Minnesota Fishes: Just How Many Species Are There Anyway?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -

Iowa Darter Etheostoma Exile ILLINOIS RANGE
Iowa darter Etheostoma exile Kingdom: Animalia FEATURES Phylum: Chordata The Iowa darter averages about two and three- Class: Osteichthyes fourths inches in length. It is a brown or green- Order: Perciformes brown fish with eight to 10 dark marks on the back and 10 to 14 dark blotches on the side separated by Family: Percidae red spaces. There is a dark, teardrop mark under the ILLINOIS STATUS eye and a dark bar in front of the eye, as well as bars on the fins. The lateral line is short, extending to common, native about the second dorsal fin. There are two spines in the anal fin. The cheeks have scales. The breeding male has a blue tint to the back, green side blotches separated by rust-red spaces, wide bands of blue and orange in the first dorsal fin and orange along the lower sides. BEHAVIORS The Iowa darter may be found in glacial lakes in northeastern Illinois, a few streams in northern Illinois and a few limestone quarries in Vermilion County. It lives in clear lakes, sloughs and creeks that have many aquatic plants. In streams it can be found in quiet pools over a mud or clay bottom with dead material and brush. Spawning occurs in April in shallow water over roots, vegetation or debris. The young Iowa darter eats plankton, while the adult feeds on immature insects and small crustaceans. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. female © Konrad P. Schmidt, University of Minnesota male © Konrad P. -

Fish Species of Saskatchewan
Introduction From the shallow, nutrient -rich potholes of the prairies to the clear, cool rock -lined waters of our province’s north, Saskatchewan can boast over 50,000 fish-bearing bodies of water. Indeed, water accounts for about one-eighth, or 80,000 square kilometers, of this province’s total surface area. As numerous and varied as these waterbodies are, so too are the types of fish that inhabit them. In total, Saskatchewan is home to 67 different fish species from 16 separate taxonomic families. Of these 67, 58 are native to Saskatchewan while the remaining nine represent species that have either been introduced to our waters or have naturally extended their range into the province. Approximately one-third of the fish species found within Saskatchewan can be classed as sportfish. These are the fish commonly sought out by anglers and are the best known. The remaining two-thirds can be grouped as minnow or rough-fish species. The focus of this booklet is primarily on the sportfish of Saskatchewan, but it also includes information about several rough-fish species as well. Descriptions provide information regarding the appearance of particular fish as well as habitat preferences and spawning and feeding behaviours. The individual species range maps are subject to change due to natural range extensions and recessions or because of changes in fisheries management. "...I shall stay him no longer than to wish him a rainy evening to read this following Discourse; and that, if he be an honest Angler, the east wind may never blow when he goes a -fishing." The Compleat Angler Izaak Walton, 1593-1683 This booklet was originally published by the Saskatchewan Watershed Authority with funds generated from the sale of angling licences and made available through the FISH AND WILDLIFE DEVELOPMENT FUND. -

Northern Pike Esox Lucius ILLINOIS RANGE
northern pike Esox lucius Kingdom: Animalia FEATURES Phylum: Chordata The northern pike's average life span is eight to 10 Class: Osteichthyes years. The average weight is two pounds. It may Order: Esociformes attain a maximum length of 53 inches. The fins are rounded, and all except the pectoral fins have dark Family: Esocidae spots. Scales are present on the cheek and half of ILLINOIS STATUS the gill cover. The eyes are yellow. The long, green body has yellow spots on the sides. The belly is common, native white to dark yellow. The duckbill-shaped snout is easily seen. BEHAVIORS The northern pike lives in lakes, rivers and marshes. It prefers water without strong currents and with many plants. This fish reaches maturity at age two to three years. Spawning occurs in March. The female deposits up to 150,000 eggs that are scattered in marshy areas or other shallow water areas. Eggs hatch in 12-14 days. This fish eats fishes, insects, crayfish, frogs and reptiles. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. close up of head close up of side © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. © Engbretson Underwater Photography adult Aquatic Habitats lakes, ponds and reservoirs; rivers and streams; marshes Woodland Habitats none Prairie and Edge Habitats none © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources.. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -
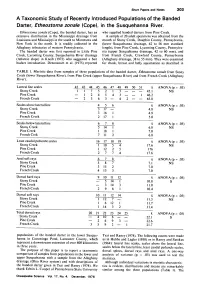
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Fisheries Full Issue PDF Volume 39, Issue 3
This article was downloaded by: [American Fisheries Society] On: 24 March 2014, At: 13:30 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Fisheries Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ufsh20 Full Issue PDF Volume 39, Issue 3 Published online: 21 Mar 2014. To cite this article: (2014) Full Issue PDF Volume 39, Issue 3, Fisheries, 39:3, 97-144, DOI: 10.1080/03632415.2014.902705 To link to this article: http://dx.doi.org/10.1080/03632415.2014.902705 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. This article may be used for research, teaching, and private study purposes. -

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -

Esox Lucius) Ecological Risk Screening Summary
Northern Pike (Esox lucius) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2019 Web Version, 8/26/2019 Photo: Ryan Hagerty/USFWS. Public Domain – Government Work. Available: https://digitalmedia.fws.gov/digital/collection/natdiglib/id/26990/rec/22. (February 1, 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019a): “Circumpolar in fresh water. North America: Atlantic, Arctic, Pacific, Great Lakes, and Mississippi River basins from Labrador to Alaska and south to Pennsylvania and Nebraska, USA [Page and Burr 2011]. Eurasia: Caspian, Black, Baltic, White, Barents, Arctic, North and Aral Seas and Atlantic basins, southwest to Adour drainage; Mediterranean basin in Rhône drainage and northern Italy. Widely distributed in central Asia and Siberia easward [sic] to Anadyr drainage (Bering Sea basin). Historically absent from Iberian Peninsula, Mediterranean France, central Italy, southern and western Greece, eastern Adriatic basin, Iceland, western Norway and northern Scotland.” Froese and Pauly (2019a) list Esox lucius as native in Armenia, Azerbaijan, China, Georgia, Iran, Kazakhstan, Mongolia, Turkey, Turkmenistan, Uzbekistan, Albania, Austria, Belgium, Bosnia Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Moldova, Monaco, 1 Netherlands, Norway, Poland, Romania, Russia, Serbia, Slovakia, Slovenia, Sweden, Switzerland, United Kingdom, Ukraine, Canada, and the United States (including Alaska). From Froese and Pauly (2019a): “Occurs in Erqishi river and Ulungur lake [in China].” “Known from the Selenge drainage [in Mongolia] [Kottelat 2006].” “[In Turkey:] Known from the European Black Sea watersheds, Anatolian Black Sea watersheds, Central and Western Anatolian lake watersheds, and Gulf watersheds (Firat Nehri, Dicle Nehri). -

Ruffe (Gymnocephalus Cernua) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Ruffe (Gymnocephalus cernua) Ecological Risk Screening Summary US Fish and Wildlife Service, February 2011 Revised, July 2014 Revised, June 2015 Photo: USFWS 1 Native Range, and Status in the United States Native Range From Fuller et al. (2014): “Northern Europe and Asia (Berg 1949; Holcik and Hensel 1974; Wheeler 1978; Page and Burr 1991).” Status in the United States From Fuller et al. (2014): “The ruffe was first identified by Wisconsin DNR in specimens collected from the St. Louis River at the border of Minnesota and Wisconsin in 1987 (Pratt 1988; Pratt et al. 1992; Czypinski et al. 1999, 2000, 2001, 2003). Following that report, reexamination of archived samples revealed misidentified larval specimens of ruffe had been collected from the same area in 1986 (Pratt 1988). The ruffe subsequently spread into Duluth Harbor in Lake Superior and several tributaries of the lake (Underhill 1989; Czypinski et al. 1999, 2000, 2004; Scheidegger, pers. comm.; J. Slade, pers. comm.). It is found in the Amnicon, Flag, Iron, Middle, Raspberry, and Bad rivers, Chequamegon Bay, and Apostle Islands National Lakeshore in Wisconsin (Czypinski et al. 1999, 2000, 2001, 2003, 2004; Tilmant 1999). In August 1994, it was found in Saxon Harbor, Wisconsin, and in the upper peninsula of Michigan at the mouths of the Black and Ontonagon rivers (K. Kindt, pers. comm.). In the lower Peninsula of Michigan along Lake Huron, the first three specimens were caught at the mouth of the Thunder Bay River in August 1995 (K. Kindt, pers. comm.). This species has also been collected in Michigan in Lake Michigan, Lake Superior, Torch Lake, Little Bay de Noc in Escanaba, Big Bay de Noc, Misery River, Ontonagon River, Thunder Bay, and Sturgeon River Sloughs (Czypinski et al. -

Low-Head Dams Facilitate Round Goby Neogobius Melanostomus Invasion
Biol Invasions (2018) 20:757–776 https://doi.org/10.1007/s10530-017-1573-3 ORIGINAL PAPER Low-head dams facilitate Round Goby Neogobius melanostomus invasion Dustin Raab . Nicholas E. Mandrak . Anthony Ricciardi Received: 9 July 2017 / Accepted: 23 September 2017 / Published online: 3 October 2017 Ó Springer International Publishing AG 2017 Abstract Round Goby Neogobius melanostomus inclusion of both reservoir-associated abiotic variables invasion of the Grand River (Ontario, Canada) and Round Goby abundance as model terms. To presents an opportunity to assess the role of abiotic determine establishment potential of the uninvaded gradients in mediating the establishment and impact of reach immediately upstream, four environmental nonnative benthic fishes in rivers. In this system, habitat characteristics were used in discriminant sequential low-head dams delineate uninvaded and function analysis (DFA) to predict three potential invaded river reaches and create upstream gradients of outcomes of introduction: non-invaded and either increasing water velocity. We hypothesized that flow lower or higher Round Goby abundance (low and high refugia created by impounded reservoirs above low- invasion status, respectively) than the median number head dams enhance local Round Goby abundance. of Round Goby at invaded sites. Our DFA function Round Goby influence on the native fish community correctly classified non-invaded and high-abundance was determined by variance partitioning, and we used invasion status sites [ 85% of the time, with lower generalized additive models to identify small-bodied (73%) success in classifying low-abundance invasion benthic fish species most likely to be impacted by status sites, and the spatial pattern of our results Round Goby invasion. -

AN ANALYSIS of SEXUAL DIMORPHISM in LENGTH and WEIGHT RELATIONSHIPS of IOWA DARTERS Margaret a Harings Northland College, CB: 853 1411 Ellis Ave Ashland, WI 54806
AN ANALYSIS OF SEXUAL DIMORPHISM IN LENGTH AND WEIGHT RELATIONSHIPS OF IOWA DARTERS Margaret A Harings Northland College, CB: 853 1411 Ellis Ave Ashland, WI 54806 ABSTRACT Iowa darters, Etheostoma exile, are commonly found throughout the Lake Superior watershed. However, given their abundance and non-game status, little is known about basic life history characteristics of these fish. Iowa darters were seined from Inch Lake (Bayfield County, WI) in May of 2010 and 2011 to assess sexual dimorphism in sizes. Collected fish were immediately frozen and later thawed and measured for total length (TL) and total weight (TW), and dissected to remove the gonads which were also weighed. Somatic weight (SW) was calculated for each fish. The mean TL of males and females did not differ. There was a weak difference in the relationships between log(TW) and log(TL) between male and female Iowa darters. There was a strong difference in the relationships between the log(GW) and log(TL) for males and females. However, a significant difference did not exist between log(somatic weight) and log(TL) between males and females. The weak difference in the TL-TW relationship between males and females appear to be due to the strong difference in the TL-GW relationship between the sexes. Additional research is planned to determine whether differences between males and females occur in age structure and diet. INTRODUCTION Iowa darters are small fish that are typically found in clear to slightly turbid, light brown water of small lakes, bogs, and small streams (Becker 1983). The range of Iowa darters extends east to New York, west to Montana, north to southern Canada, and south to portions of Illinois (Scott and Crossman 1973; Lee and Gilbert 1978).