Comparative Growth, Reproduction, Habitat and Food Utilization Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Day Versus Night Electrofishing Catches from Near-Shore Waters of the Ohio and Muskingum Rivers1
Day Versus Night Electrofishing Catches from Near-Shore Waters of the Ohio and Muskingum Rivers1 RANDALL E. SANDERS, Division of Water Quality Planning and Assessment, State of Ohio Environmental Protection Agency, 1685 Westbelt Drive, Columbus, OH 43228 ABSTRACT. Day and night electrofishing catches were compared for sampling effectiveness and diel movements offish to and from near-shore waters of the Ohio and Muskingum rivers. Standardized methods were used to collect same-day paired samples by sampling during the day, displacing the catch, and resampling after twilight. Night catches contained significantly higher numbers of species, individuals (excluding Dorosoma cepedianum), weight, and biological index scores (Modified Index of Well-Being [Mlwb] and Index of Biotic Integrity [IBI]). Night versus day paired samples in the Ohio and Muskingum rivers showed, respectively, mean increases of 7.6 and 4.6 species, 229 and 417 fish per km (excluding D. cepedianum), 18.2 and 30.4 kg/km, 2.3 and 1.5 Mlwb units, and 10.8 and 8.7 IBI units. Total night catches yielded, respectively, 43% and 15% more taxa, 62% and 160% greater numbers (excluding!), cepedianum), and 50% and 70% more weight than total day catches. Catch differences were primarily attributed to diel movements from off-shore to near-shore waters during the evening-twilight period. Taxa which increased the most at night in the Ohio River were: Alosa chrysochloris, Notropis wicklijfi, Ictiobus bubalus, Moxostoma anisurum, M. duquesnei, Ictalurus punctatus, Morone saxatilis x M. chrysops, Ambloplites rupestris, Stizostedion canadense, and Aplodinotus grunniens; and in the Muskingum River: Ictiobus bubalus, Moxostoma anisurum, and Morone chrysops. -

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -

Percina Copelandi) in CANADA
Canadian Science Advisory Secretariat Central and Arctic, and Quebec Regions Science Advisory Report 2010/058 RECOVERY POTENTIAL ASSESSMENT OF CHANNEL DARTER (Percina copelandi) IN CANADA Channel Darter (Percina copelandi) Figure 1. Distribution of Channel Darter in Canada. © Ellen Edmondson Context : The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assessed the status of Channel Darter (Percina copelandi) in April 1993. The assessment resulted in the designation of Channel Darter as Threatened. In May 2002, the status was re-examined and confirmed by COSEWIC. This designation was assigned because the species exists in low numbers where found, and its habitat is negatively impacted by siltation and fluctuations in water levels. Subsequent to the COSEWIC designation, Channel Darter was included on Schedule 1 of the Species at Risk Act (SARA) when the Act was proclaimed in June 2003. A species Recovery Potential Assessment (RPA) process has been developed by Fisheries and Oceans Canada (DFO) Science to provide the information and scientific advice required to meet the various requirements of the SARA, such as the authorization to carry out activities that would otherwise violate the SARA as well as the development of recovery strategies. The scientific information also serves as advice to the Minister of DFO regarding the listing of the species under SARA and is used when analyzing the socio-economic impacts of adding the species to the list as well as during subsequent consultations, where applicable. This assessment considers the scientific data available with which to assess the recovery potential of Channel Darter in Canada. SUMMARY In Ontario, the current and historic Channel Darter distribution is limited to four distinct areas of the Great Lakes basin: Lake St. -

Fish Inventory at Stones River National Battlefield
Fish Inventory at Stones River National Battlefield Submitted to: Department of the Interior National Park Service Cumberland Piedmont Network By Dennis Mullen Professor of Biology Department of Biology Middle Tennessee State University Murfreesboro, TN 37132 September 2006 Striped Shiner (Luxilus chrysocephalus) – nuptial male From Lytle Creek at Fortress Rosecrans Photograph by D. Mullen Table of Contents List of Tables……………………………………………………………………….iii List of Figures………………………………………………………………………iv List of Appendices…………………………………………………………………..v Executive Summary…………………………………………………………………1 Introduction…………………………………………………………………...……..2 Methods……………………………………………………………………………...3 Results……………………………………………………………………………….7 Discussion………………………………………………………………………….10 Conclusions………………………………………………………………………...14 Literature Cited…………………………………………………………………….15 ii List of Tables Table1: Location and physical characteristics (during September 2006, and only for the riverine sites) of sample sites for the STRI fish inventory………………………………17 Table 2: Biotic Integrity classes used in assessing fish communities along with general descriptions of their attributes (Karr et al. 1986) ………………………………………18 Table 3: List of fishes potentially occurring in aquatic habitats in and around Stones River National Battlefield………………………………………………………………..19 Table 4: Fish species list (by site) of aquatic habitats at STRI (October 2004 – August 2006). MF = McFadden’s Ford, KP = King Pond, RB = Redoubt Brannan, UP = Unnamed Pond at Redoubt Brannan, LC = Lytle Creek at Fortress Rosecrans……...….22 Table 5: Fish Species Richness estimates for the 3 riverine reaches of STRI and a composite estimate for STRI as a whole…………………………………………………24 Table 6: Index of Biotic Integrity (IBI) scores for three stream reaches at Stones River National Battlefield during August 2005………………………………………………...25 Table 7: Temperature and water chemistry of four of the STRI sample sites for each sampling date…………………………………………………………………………….26 Table 8 : Total length estimates of specific habitat types at each riverine sample site. -

Darter Reproductive Seasons Author(S): Clark Hubbs Reviewed Work(S): Source: Copeia, Vol
Darter Reproductive Seasons Author(s): Clark Hubbs Reviewed work(s): Source: Copeia, Vol. 1985, No. 1 (Feb. 11, 1985), pp. 56-68 Published by: American Society of Ichthyologists and Herpetologists (ASIH) Stable URL: http://www.jstor.org/stable/1444790 . Accessed: 10/01/2012 14:26 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. American Society of Ichthyologists and Herpetologists (ASIH) is collaborating with JSTOR to digitize, preserve and extend access to Copeia. http://www.jstor.org 56 COPEIA, 1985, NO. 1 changes in kinosternid turtles. J. Herpetol. 6:183- . 1938. Seasonal changes in the testes of the 189. musk turtle Sternotherusodoratus L. J. Morphol. 63: MCPHERSON, R. J., AND K. R. MARION. 1981. Sea- 301-317. sonal testicular cycle of the stinkpot turtle (Ster- SAINTGIRONS, H. 1982. Reproductive cycles of male notherus odoratus) in central Alabama. Herpetolog- snakes and their relationships with climate and fe- ica 37:33-40. male reproductive cycles. Herpetologica 38:5-16. MITCHELL, J. C. 1982. Population ecology and de- SPEAT, R. H. 1973. Seasonal variation in the tubular mography of the freshwater turtles Chrysemyspicta and interstitial areas of the testes in Sternothaerus and Sternotherusodoratus. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -
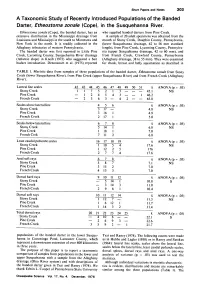
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Kansas Stream Fishes
A POCKET GUIDE TO Kansas Stream Fishes ■ ■ ■ ■ ■ ■ ■ ■ ■ ■ By Jessica Mounts Illustrations © Joseph Tomelleri Sponsored by Chickadee Checkoff, Westar Energy Green Team, Kansas Department of Wildlife, Parks and Tourism, Kansas Alliance for Wetlands & Streams, and Kansas Chapter of the American Fisheries Society Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Fish Anatomy • 3 • Species Accounts: Sturgeons (Family Acipenseridae) • 4 ■ Shovelnose Sturgeon • 5 ■ Pallid Sturgeon • 6 Minnows (Family Cyprinidae) • 7 ■ Southern Redbelly Dace • 8 ■ Western Blacknose Dace • 9 ©Ryan Waters ■ Bluntface Shiner • 10 ■ Red Shiner • 10 ■ Spotfin Shiner • 11 ■ Central Stoneroller • 12 ■ Creek Chub • 12 ■ Peppered Chub / Shoal Chub • 13 Plains Minnow ■ Silver Chub • 14 ■ Hornyhead Chub / Redspot Chub • 15 ■ Gravel Chub • 16 ■ Brassy Minnow • 17 ■ Plains Minnow / Western Silvery Minnow • 18 ■ Cardinal Shiner • 19 ■ Common Shiner • 20 ■ Bigmouth Shiner • 21 ■ • 21 Redfin Shiner Cover Photo: Photo by Ryan ■ Carmine Shiner • 22 Waters. KDWPT Stream ■ Golden Shiner • 22 Survey and Assessment ■ Program collected these Topeka Shiner • 23 male Orangespotted Sunfish ■ Bluntnose Minnow • 24 from Buckner Creek in Hodgeman County, Kansas. ■ Bigeye Shiner • 25 The fish were catalogued ■ Emerald Shiner • 26 and returned to the stream ■ Sand Shiner • 26 after the photograph. ■ Bullhead Minnow • 27 ■ Fathead Minnow • 27 ■ Slim Minnow • 28 ■ Suckermouth Minnow • 28 Suckers (Family Catostomidae) • 29 ■ River Carpsucker • -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

BREAK-OUT SESSIONS at a GLANCE THURSDAY, 24 JULY, Afternoon Sessions
2008 Joint Meeting (JMIH), Montreal, Canada BREAK-OUT SESSIONS AT A GLANCE THURSDAY, 24 JULY, Afternoon Sessions ROOM Salon Drummond West & Center Salons A&B Salons 6&7 SESSION/ Fish Ecology I Herp Behavior Fish Morphology & Histology I SYMPOSIUM MODERATOR J Knouft M Whiting M Dean 1:30 PM M Whiting M Dean Can She-male Flat Lizards (Platysaurus broadleyi) use Micro-mechanics and material properties of the Multiple Signals to Deceive Male Rivals? tessellated skeleton of cartilaginous fishes 1:45 PM J Webb M Paulissen K Conway - GDM The interopercular-preopercular articulation: a novel Is prey detection mediated by the widened lateral line Variation In Spatial Learning Within And Between Two feature suggesting a close relationship between canal system in the Lake Malawi cichlid, Aulonocara Species Of North American Skinks Psilorhynchus and labeonin cyprinids (Ostariophysi: hansbaenchi? Cypriniformes) 2:00 PM I Dolinsek M Venesky D Adriaens Homing And Straying Following Experimental Effects of Batrachochytrium dendrobatidis infections on Biting for Blood: A Novel Jaw Mechanism in Translocation Of PIT Tagged Fishes larval foraging performance Haematophagous Candirú Catfish (Vandellia sp.) 2:15 PM Z Benzaken K Summers J Bagley - GDM Taxonomy, population genetics, and body shape The tale of the two shoals: How individual experience A Key Ecological Trait Drives the Evolution of Monogamy variation of Alabama spotted bass Micropterus influences shoal behaviour in a Peruvian Poison Frog punctulatus henshalli 2:30 PM M Pyron K Parris L Chapman