Underwater Observation and Habitat Utilization of Three Rare Darters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Funding (Total $2,552,481) $15,000 2019
CURRICULUM VITAE TENNESSEE AQUARIUM CONSERVATION INSTITUTE 175 BAYLOR SCHOOL RD CHATTANOOGA, TN 37405 RESEARCH FUNDING (TOTAL $2,552,481) $15,000 2019. Global Wildlife Conservation. Rediscovering the critically endangered Syr-Darya Shovelnose Sturgeon. $10,000 2019. Tennessee Wildlife Resources Agency. Propagation of the Common Logperch as a host for endangered mussel larvae. $8,420 2019. Tennessee Wildlife Resources Agency. Monitoring for the Laurel Dace. $4,417 2019. Tennessee Wildlife Resources Agency. Examining interactions between Laurel Dace (Chrosomus saylori) and sunfish $12,670 2019. Trout Unlimited. Southern Appalachian Brook Trout propagation for reintroduction to Shell Creek. $106,851 2019. Private Donation. Microplastic accumulation in fishes of the southeast. $1,471. 2019. AZFA-Clark Waldram Conservation Grant. Mayfly propagation for captive propagation programs. $20,000. 2019. Tennessee Valley Authority. Assessment of genetic diversity within Blotchside Logperch. $25,000. 2019. Riverview Foundation. Launching Hidden Rivers in the Southeast. $11,170. 2018. Trout Unlimited. Propagation of Southern Appalachian Brook Trout for Supplemental Reintroduction. $1,471. 2018. AZFA Clark Waldram Conservation Grant. Climate Change Impacts on Headwater Stream Vertebrates in Southeastern United States $1,000. 2018. Hamilton County Health Department. Step 1 Teaching Garden Grants for Sequoyah School Garden. $41,000. 2018. Riverview Foundation. River Teachers: Workshops for Educators. $1,000. 2018. Tennessee Valley Authority. Youth Freshwater Summit $20,000. 2017. Tennessee Valley Authority. Lake Sturgeon Propagation. $7,500 2017. Trout Unlimited. Brook Trout Propagation. $24,783. 2017. Tennessee Wildlife Resource Agency. Assessment of Percina macrocephala and Etheostoma cinereum populations within the Duck River Basin. $35,000. 2017. U.S. Fish and Wildlife Service. Status surveys for conservation status of Ashy (Etheostoma cinereum) and Redlips (Etheostoma maydeni) Darters. -

Percina Copelandi) in CANADA
Canadian Science Advisory Secretariat Central and Arctic, and Quebec Regions Science Advisory Report 2010/058 RECOVERY POTENTIAL ASSESSMENT OF CHANNEL DARTER (Percina copelandi) IN CANADA Channel Darter (Percina copelandi) Figure 1. Distribution of Channel Darter in Canada. © Ellen Edmondson Context : The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assessed the status of Channel Darter (Percina copelandi) in April 1993. The assessment resulted in the designation of Channel Darter as Threatened. In May 2002, the status was re-examined and confirmed by COSEWIC. This designation was assigned because the species exists in low numbers where found, and its habitat is negatively impacted by siltation and fluctuations in water levels. Subsequent to the COSEWIC designation, Channel Darter was included on Schedule 1 of the Species at Risk Act (SARA) when the Act was proclaimed in June 2003. A species Recovery Potential Assessment (RPA) process has been developed by Fisheries and Oceans Canada (DFO) Science to provide the information and scientific advice required to meet the various requirements of the SARA, such as the authorization to carry out activities that would otherwise violate the SARA as well as the development of recovery strategies. The scientific information also serves as advice to the Minister of DFO regarding the listing of the species under SARA and is used when analyzing the socio-economic impacts of adding the species to the list as well as during subsequent consultations, where applicable. This assessment considers the scientific data available with which to assess the recovery potential of Channel Darter in Canada. SUMMARY In Ontario, the current and historic Channel Darter distribution is limited to four distinct areas of the Great Lakes basin: Lake St. -

Finding Aid for Clark Hubbs Archive
Inventory of the Clark Hubbs Papers, 1946-1999 The University of Texas at Austin – Texas Natural History Collections, Ichthyology Collection Completed by Sara D’Antonio - August 12, 2011 Extent: 24.5 linear feet Restrictions Boxes 57 and 58 contain restricted materials under the Family Educational Rights and Privacy Act (FERPA) and Health Insurance Portability and Accountability Act (HIPAA). Abstract Dr. Clark Hubbs was a professor in the School of Biological Sciences, Section of Integrative Biology at The University of Texas at Austin, for his entire career, from 1949 until his death in 2008. He founded the University’s Fish Collection, which is now part of the Texas Natural History Collections and deposited more fish specimens than anyone else has, or likely ever will. Hubbs published over 300 articles during his career and his idea for a book on the fishes of Texas began the Fishes of Texas Project. The Clark Hubbs Papers measures 24.5 linear feet and includes research notes, reprints, field notes, manuscripts, and some student records dating from 1946-1999. History Dr. Clark Hubbs was born March 15, 1921, in Ann Arbor, Michigan, to Carl Leavitt Hubbs and Laura Cornelia Clark Hubbs. His father was a noted ichthyologist, and the family revolved around fish. On vacations the family did fieldwork and all the Hubbs children were paid for collecting specimens: five cents per species, one dollar for a new species or subspecies, and five dollars for a new genus. Hubbs received his B.A. in Zoology from the University of Michigan in 1942, shortly after he was drafted into the army. -

A Thesis Entitled Molecular, Morphological, and Biogeographic Resolution of Cryptic Taxa in the Greenside Darter Etheostoma Blen
A Thesis Entitled Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex By Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) ____________________________ Advisor: Dr. Carol A. Stepien ____________________________ Committee Member: Dr. Timothy G. Fisher ____________________________ Committee Member: Dr. Johan F. Gottgens ____________________________ College of Graduate Studies The University of Toledo December 2007 Copyright © 2007 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) The University of Toledo December 2007 DNA sequencing has led to the resolution of many cryptic taxa, which are especially prevalent in the North American darter fishes (Family Percidae). The Greenside Darter Etheostoma blennioides commonly occurs in the lower Great Lakes region, where two putative subspecies, the eastern “Allegheny” type E. b. blennioides and the western “Prairie” type E. b. pholidotum , overlap. The objective of this study was to test the systematic identity and genetic divergence distinguishing the two subspecies in areas of sympatry and allopatry in comparison to other subspecies and close relatives. DNA sequences from the mtDNA cytochrome b gene and control region and the nuclear S7 intron 1 comprising a total of 1,497 bp were compared from 294 individuals across 18 locations, including the Lake Erie basin and the Allegheny, Meramec, Obey, Ohio, Rockcastle, Susquehanna, and Wabash River systems. -

C:\Fish\Eastern Sand Darter Sa.Wpd
EASTERN SAND DARTER STATUS ASSESSMENT Prepared by: David Grandmaison and Joseph Mayasich Natural Resources Research Institute University of Minnesota 5013 Miller Trunk Highway Duluth, MN 55811-1442 and David Etnier Ecology and Evolutionary Biology University of Tennessee 569 Dabney Hall Knoxville, TN 37996-1610 Prepared for: U.S. Fish and Wildlife Service Region 3 1 Federal Drive Fort Snelling, MN 55111 January 2004 NRRI Technical Report No. NRRI/TR-2003/40 DISCLAIMER This document is a compilation of biological data and a description of past, present, and likely future threats to the eastern sand darter, Ammocrypta pellucida (Agassiz). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://midwest.fws.gov/eco_serv/endangrd/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. ii TABLE OF CONTENTS DISCLAIMER................................................................... -

Lessons from the Tellico Dam Controversy
Volume 43 Issue 4 Fall 2003 Fall 2003 Public Goods Provision: Lessons from the Tellico Dam Controversy Louis P. Cain Brooks A. Kaiser Recommended Citation Louis P. Cain & Brooks A. Kaiser, Public Goods Provision: Lessons from the Tellico Dam Controversy, 43 Nat. Resources J. 979 (2003). Available at: https://digitalrepository.unm.edu/nrj/vol43/iss4/4 This Article is brought to you for free and open access by the Law Journals at UNM Digital Repository. It has been accepted for inclusion in Natural Resources Journal by an authorized editor of UNM Digital Repository. For more information, please contact [email protected], [email protected], [email protected]. LOUIS P. CAIN & BROOKS A. KAISER* Public Goods Provision: Lessons from the Tellico Dam Controversy ABSTRACT Although absent from the initial Endangered Species Act (ESA) of 1973, evidence of economic considerationsfirst appeared in the 1978 amendments. The only controversial vote concerning the ESA was the one to exempt the Tellico Dam (1978). Although the dam was a local project with little expected net benefit, this article argues that broader economic considerations mattered. Working from public choice models for congressional voting decisions, a limited dependent variable regression analysis indicates the economic variables with the most explanatory power for this environmental decision are college education, poverty, the designation of critical habitat within a district, the number of endangered species in the state, dollars the state received due to earlier ESA funding, and the percentage of the district that is federal land. Comparisons with aggregated environmental votes in the same year highlight the intensity of economic considerations in the Tellico case. -

Darter Reproductive Seasons Author(S): Clark Hubbs Reviewed Work(S): Source: Copeia, Vol
Darter Reproductive Seasons Author(s): Clark Hubbs Reviewed work(s): Source: Copeia, Vol. 1985, No. 1 (Feb. 11, 1985), pp. 56-68 Published by: American Society of Ichthyologists and Herpetologists (ASIH) Stable URL: http://www.jstor.org/stable/1444790 . Accessed: 10/01/2012 14:26 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. American Society of Ichthyologists and Herpetologists (ASIH) is collaborating with JSTOR to digitize, preserve and extend access to Copeia. http://www.jstor.org 56 COPEIA, 1985, NO. 1 changes in kinosternid turtles. J. Herpetol. 6:183- . 1938. Seasonal changes in the testes of the 189. musk turtle Sternotherusodoratus L. J. Morphol. 63: MCPHERSON, R. J., AND K. R. MARION. 1981. Sea- 301-317. sonal testicular cycle of the stinkpot turtle (Ster- SAINTGIRONS, H. 1982. Reproductive cycles of male notherus odoratus) in central Alabama. Herpetolog- snakes and their relationships with climate and fe- ica 37:33-40. male reproductive cycles. Herpetologica 38:5-16. MITCHELL, J. C. 1982. Population ecology and de- SPEAT, R. H. 1973. Seasonal variation in the tubular mography of the freshwater turtles Chrysemyspicta and interstitial areas of the testes in Sternothaerus and Sternotherusodoratus. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -
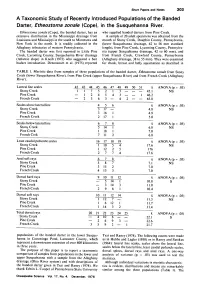
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Kansas Stream Fishes
A POCKET GUIDE TO Kansas Stream Fishes ■ ■ ■ ■ ■ ■ ■ ■ ■ ■ By Jessica Mounts Illustrations © Joseph Tomelleri Sponsored by Chickadee Checkoff, Westar Energy Green Team, Kansas Department of Wildlife, Parks and Tourism, Kansas Alliance for Wetlands & Streams, and Kansas Chapter of the American Fisheries Society Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Fish Anatomy • 3 • Species Accounts: Sturgeons (Family Acipenseridae) • 4 ■ Shovelnose Sturgeon • 5 ■ Pallid Sturgeon • 6 Minnows (Family Cyprinidae) • 7 ■ Southern Redbelly Dace • 8 ■ Western Blacknose Dace • 9 ©Ryan Waters ■ Bluntface Shiner • 10 ■ Red Shiner • 10 ■ Spotfin Shiner • 11 ■ Central Stoneroller • 12 ■ Creek Chub • 12 ■ Peppered Chub / Shoal Chub • 13 Plains Minnow ■ Silver Chub • 14 ■ Hornyhead Chub / Redspot Chub • 15 ■ Gravel Chub • 16 ■ Brassy Minnow • 17 ■ Plains Minnow / Western Silvery Minnow • 18 ■ Cardinal Shiner • 19 ■ Common Shiner • 20 ■ Bigmouth Shiner • 21 ■ • 21 Redfin Shiner Cover Photo: Photo by Ryan ■ Carmine Shiner • 22 Waters. KDWPT Stream ■ Golden Shiner • 22 Survey and Assessment ■ Program collected these Topeka Shiner • 23 male Orangespotted Sunfish ■ Bluntnose Minnow • 24 from Buckner Creek in Hodgeman County, Kansas. ■ Bigeye Shiner • 25 The fish were catalogued ■ Emerald Shiner • 26 and returned to the stream ■ Sand Shiner • 26 after the photograph. ■ Bullhead Minnow • 27 ■ Fathead Minnow • 27 ■ Slim Minnow • 28 ■ Suckermouth Minnow • 28 Suckers (Family Catostomidae) • 29 ■ River Carpsucker •