PRIMARY HOMOTHALLISM-RELATION to HETEROTHALLISM in the REGULATION of SEXUAL MORPHOGENESIS in Sistotremal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Self-Fertility and Uni-Directional Mating-Type Switching in Ceratocystis Coerulescens, a Filamentous Ascomycete
Curr Genet (1997) 32: 52–59 © Springer-Verlag 1997 ORIGINAL PAPER T. C. Harrington · D. L. McNew Self-fertility and uni-directional mating-type switching in Ceratocystis coerulescens, a filamentous ascomycete Received: 6 July 1996 / 25 March 1997 Abstract Individual perithecia from selfings of most some filamentous ascomycetes. Although a switch in the Ceratocystis species produce both self-fertile and self- expression of mating-type is seen in these fungi, it is not sterile progeny, apparently due to uni-directional mating- clear if a physical movement of mating-type genes is in- type switching. In C. coerulescens, male-only mutants of volved. It is also not clear if the expressed mating-types otherwise hermaphroditic and self-fertile strains were self- of the respective self-fertile and self-sterile progeny are sterile and were used in crossings to demonstrate that this homologs of the mating-type genes in other strictly heter- species has two mating-types. Only MAT-2 strains are othallic species of ascomycetes. capable of selfing, and half of the progeny from a MAT-2 Sclerotinia trifoliorum and Chromocrea spinulosa show selfing are MAT-1. Male-only, MAT-2 mutants are self- a 1:1 segregation of self-fertile and self-sterile progeny in sterile and cross only with MAT-1 strains. Similarly, self- perithecia from selfings or crosses (Mathieson 1952; Uhm fertile strains generally cross with only MAT-1 strains. and Fujii 1983a, b). In tetrad analyses of selfings or crosses, MAT-1 strains only cross with MAT-2 strains and never self. half of the ascospores in an ascus are large and give rise to It is hypothesized that the switch in mating-type during self-fertile colonies, and the other ascospores are small and selfing is associated with a deletion of the MAT-2 gene. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

Heterothallism and Potential Hybridization Events Inferred For
Du et al. IMA Fungus (2020) 11:4 https://doi.org/10.1186/s43008-020-0027-1 IMA Fungus RESEARCH Open Access Heterothallism and potential hybridization events inferred for twenty-two yellow morel species Xi-Hui Du1* , Dongmei Wu2, Heng Kang3*, Hanchen Wang1, Nan Xu1, Tingting Li1 and Keliang Chen1 Abstract Mating-type genes are central to sexual reproduction in ascomycete fungi and result in the establishment of reproductive barriers. Together with hybridization, they both play important roles in the evolution of fungi. Recently, potential hybridization events and MAT genes were separately found in the Elata Clade of Morchella. Herein, we characterized the MAT1–1-1 and MAT1–2-1 genes of twenty-two species in the Esculenta Clade, another main group in the genus Morchella, and proved heterothallism to be the predominant mating strategy among the twenty-two species tested. Ascospores of these species were multi-nuclear and had many mitochondrial nucleoids. The number of ascospore nuclei might be positively related with the species distribution range. Phylogenetic analyses of MAT1–1-1, MAT1–2-1, intergenic spacer (IGS), and partial histone acetyltransferase ELP3 (F1) were performed and compared with the species phylogeny framework derived from the ribosomal internal transcribed spacer region (ITS) and translation elongation factor 1-alpha (EF1-a) to evaluate their species delimitation ability and investigate potential hybridization events. Conflicting topologies among these genes genealogies and the species phylogeny were revealed and hybridization events were detected between several species. Different evolutionary patterns were suggested for MAT genes between the Esculenta and the Elata Clades. Complex evolutionary trajectories of MAT1–1-1, MAT1–2-1, F1 andIGSintheEsculentaCladewerehighlighted.Thesefindings contribute to a better understanding of the importance of hybridization and gene transfer in Morchella andespeciallyfortheappearanceofreproductivemodesduring its evolutionary process. -
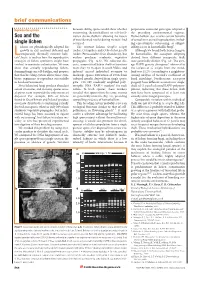
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

Discovery of a Sexual Cycle in Aspergillus Lentulus, a Close Relative of A
Discovery of a Sexual Cycle in Aspergillus lentulus, a Close Relative of A. fumigatus Sameira S. Swilaiman,a Céline M. O’Gorman,a S. Arunmozhi Balajee,b Paul S. Dyera School of Biology, University of Nottingham, University Park, Nottingham, United Kingdoma; Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USAb Aspergillus lentulus was described in 2005 as a new species within the A. fumigatus sensu lato complex. It is an opportunistic human pathogen causing invasive aspergillosis with high mortality rates, and it has been isolated from clinical and environmen- tal sources. The species is morphologically nearly identical to A. fumigatus sensu stricto, and this similarity has resulted in their frequent misidentification. Comparative studies show that A. lentulus has some distinguishing growth features and decreased in vitro susceptibility to several antifungal agents, including amphotericin B and caspofungin. Similar to the once-presumed-asex- ual A. fumigatus, it has only been known to reproduce mitotically. However, we now show that A. lentulus has a heterothallic sexual breeding system. A PCR-based mating-type diagnostic detected isolates of either the MAT1-1 or MAT1-2 genotype, and examination of 26 worldwide clinical and environmental isolates revealed similar ratios of the two mating types (38% versus 62%, respectively). MAT1-1 and MAT1-2 idiomorph regions were analyzed, revealing the presence of characteristic alpha and high-mobility-group (HMG) domain genes, together with other more unusual features such as a MAT1-2-4 gene. We then dem- onstrated that A. lentulus possesses a functional sexual cycle with mature cleistothecia, containing heat-resistant ascospores, being produced after 3 weeks of incubation. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

Topic: Heterothallism in Fungi
M.SC SEMESTER I : E CONTENT FOR MBOTCC-I: Phycology, Mycology and Bryology ,Unit 3 continued Faculty : Dr Tanuja, University Department of Botany, Patliputra University Topic: Heterothallism in fungi Ehrenbergh (1829), for the first time studied zygospores in the order Mucorales. The American mycologist Blakeslee (1904), reported that in the several genera of the order Mucorales, the zygospores are not formed at all. The term Heterothallism was first by A.F. Blakeslee in 1904 when he observed that zygospores could develop in some spp. only when two mycelia of different strains were allowed to come in contact with each other. According to Blakeslee (1904) Heterothallic condition is “essentially similar to that in dioecious plants and animals and although in this case the two complimentary individuals which are needed for sexual reproduction are in general not so conspicuously differentiated morphologically as in higher forms, such a morphological difference is often distinctly visible.” He also supported his view with facts and reasons, and also investigated that in the same order two types of species are found which may be named as homothallic and heterothallic species. When the two hyphae of the same mycelium produced by a single spore fuse with each other and a zygospore is developed, the species is said to be homothallic, e.g., Mucor hiemalis. Mucor mucedo and Mucor stolonifer are the typical heterothallic species. In heterothallic species the fusion can take place only among the different strained hyphae, which develop on different mycelia of different (+ and -) strains. In these species the zygospores cannot be produced by the fusion of two hyphae of the same strain. -

New Species and Changes in Fungal Taxonomy and Nomenclature
Journal of Fungi Review From the Clinical Mycology Laboratory: New Species and Changes in Fungal Taxonomy and Nomenclature Nathan P. Wiederhold * and Connie F. C. Gibas Fungus Testing Laboratory, Department of Pathology and Laboratory Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX 78229, USA; [email protected] * Correspondence: [email protected] Received: 29 October 2018; Accepted: 13 December 2018; Published: 16 December 2018 Abstract: Fungal taxonomy is the branch of mycology by which we classify and group fungi based on similarities or differences. Historically, this was done by morphologic characteristics and other phenotypic traits. However, with the advent of the molecular age in mycology, phylogenetic analysis based on DNA sequences has replaced these classic means for grouping related species. This, along with the abandonment of the dual nomenclature system, has led to a marked increase in the number of new species and reclassification of known species. Although these evaluations and changes are necessary to move the field forward, there is concern among medical mycologists that the rapidity by which fungal nomenclature is changing could cause confusion in the clinical literature. Thus, there is a proposal to allow medical mycologists to adopt changes in taxonomy and nomenclature at a slower pace. In this review, changes in the taxonomy and nomenclature of medically relevant fungi will be discussed along with the impact this may have on clinicians and patient care. Specific examples of changes and current controversies will also be given. Keywords: taxonomy; fungal nomenclature; phylogenetics; species complex 1. Introduction Kingdom Fungi is a large and diverse group of organisms for which our knowledge is rapidly expanding. -

Multiple Pathways to Homothallism in Closely Related Yeast Lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.30.320192; this version posted December 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Multiple pathways to homothallism in closely related yeast lineages in the Basidiomycota 2 3 Alexandra Cabrita1, Márcia David-Palma1*, Patrícia H. Brito1, Joseph Heitman2, Marco A. Coelho1* and 4 Paula Gonçalves1# 5 # Corresponding author: [email protected] 6 1. Applied Molecular Biosciences Unit-UCIBIO, Departamento de Ciências da Vida, Faculdade de 7 Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal 8 2. Department of Molecular Genetics and Microbiology, Duke University, Duke University Medical 9 Center, Durham, NC 27710, USA. 10 Running title: Homothallism in Cystofilobasidium 11 12 Abstract 13 Sexual reproduction in fungi relies on proteins with well-known functions encoded by the mating-type 14 (MAT) loci. In the Basidiomycota, MAT loci are often bipartite, with the P/R locus encoding pheromone 15 precursors and pheromone receptors and the HD locus encoding heterodimerizing homeodomain 16 transcription factors (Hd1/Hd2). The interplay between different alleles of these genes within a single 17 species usually generates at least two compatible mating types. However, a minority of species are 18 homothallic, reproducing sexually without an obligate need for a compatible partner. Here we examine 19 the organization and function of the MAT loci of Cystofilobasidium capitatum, a species in the order 20 Cystofilobasidiales, which is unusually rich in homothallic species. -

Sex, Outcrossing and Mating Types: Unsolved Questions in Fungi and Beyond
doi: 10.1111/j.1420-9101.2012.02495.x REVIEW Sex, outcrossing and mating types: unsolved questions in fungi and beyond S. BILLIARD*, M. LO´ PEZ-VILLAVICENCIO ,M.E.HOODà &T.GIRAUD§– *Laboratoire de Ge´ne´tique et Evolution des Populations Ve´ge´tales, UMR CNRS 8016, Universite´ des Sciences et Technologies de Lille – Lille1, Villeneuve d’Ascq Cedex, France Origine, Structure, Evolution de la Biodiversite´, UMR 7205 CNRS-MNHN, Muse´um National d’Histoire Naturelle, Paris Cedex, France àDepartment of Biology, McGuire Life Sciences Building, Amherst College, Amherst, MA, USA §Ecologie, Syste´matique et Evolution, Universite´ Paris-Sud, Orsay cedex, France –Ecologie, Syste´matique et Evolution, CNRS, Orsay cedex, France Keywords: Abstract ascomycete; Variability in the way organisms reproduce raises numerous, and still asexual reproduction; unsolved, questions in evolutionary biology. In this study, we emphasize that basidiomycete; fungi deserve a much greater emphasis in efforts to address these questions breeding systems; because of their multiple advantages as model eukaryotes. A tremendous diploid selfing; diversity of reproductive modes and mating systems can be found in fungi, gametophytic selfing; with many evolutionary transitions among closely related species. In addition, haploid selfing; fungi show some peculiarities in their mating systems that have received little mating systems; attention so far, despite the potential for providing insights into important oomycetes; evolutionary questions. In particular, selfing can occur at the haploid stage in sexual reproduction. addition to the diploid stage in many fungi, which is generally not possible in animals and plants but has a dramatic influence upon the structure of genetic systems. Fungi also present several advantages that make them tractable models for studies in experimental evolution.