Extended Temporal Gradient for the Retrograde and Anterograde Amnesia Produced by Lbotenate Entorhinal Cortex Lesions in Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? the Role of Explicit Memory and Attention in Attitude Change Matthew D
PSYCHOLOGICAL SCIENCE Research Article DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? The Role of Explicit Memory and Attention in Attitude Change Matthew D. Lieberman, Kevin N. Ochsner, Daniel T. Gilbert, and Daniel L. Schacter Harvard University Abstract—In two studies, we investigated the roles of explicit mem- CONSCIOUS CONTENT: THE ROLE OF ory and attentional resources in the process of behavior-induced atti- EXPLICIT MEMORY tude change. Although most theories of attitude change (cognitive Explicit memory refers to one’s ability to consciously recollect dissonance and self-perception theories) assume an important role for past events, behaviors, and experiences (Schacter, Chiu, & Ochsner, both mechanisms, we propose that behavior-induced attitude change 1993) and thus is a central component of most social abilities. Indeed, can be a relatively automatic process that does not require explicit people’s memory for the identities and actions of other people and memory for, or consciously controlled processing of, the discrepancy themselves forms the adhesive that gives them a continuing sense of between attitude and behavior. Using a free-choice paradigm, we place in their social world. Revising personal attitudes and beliefs in found that both amnesics and normal participants under cognitive response to a counterattitudinal behavior naturally seems to depend on load showed as much attitude change as did control participants. retrospective capacities. If Aesop’s forlorn lover of grapes could not remember that the grapes were out of reach, he would have no reason A fox saw some ripe black grapes hanging from a trellised vine. He resorted to to persuade himself of their reduced quality. -

The Three Amnesias
The Three Amnesias Russell M. Bauer, Ph.D. Department of Clinical and Health Psychology College of Public Health and Health Professions Evelyn F. and William L. McKnight Brain Institute University of Florida PO Box 100165 HSC Gainesville, FL 32610-0165 USA Bauer, R.M. (in press). The Three Amnesias. In J. Morgan and J.E. Ricker (Eds.), Textbook of Clinical Neuropsychology. Philadelphia: Taylor & Francis/Psychology Press. The Three Amnesias - 2 During the past five decades, our understanding of memory and its disorders has increased dramatically. In 1950, very little was known about the localization of brain lesions causing amnesia. Despite a few clues in earlier literature, it came as a complete surprise in the early 1950’s that bilateral medial temporal resection caused amnesia. The importance of the thalamus in memory was hardly suspected until the 1970’s and the basal forebrain was an area virtually unknown to clinicians before the 1980’s. An animal model of the amnesic syndrome was not developed until the 1970’s. The famous case of Henry M. (H.M.), published by Scoville and Milner (1957), marked the beginning of what has been called the “golden age of memory”. Since that time, experimental analyses of amnesic patients, coupled with meticulous clinical description, pathological analysis, and, more recently, structural and functional imaging, has led to a clearer understanding of the nature and characteristics of the human amnesic syndrome. The amnesic syndrome does not affect all kinds of memory, and, conversely, memory disordered patients without full-blown amnesia (e.g., patients with frontal lesions) may have impairment in those cognitive processes that normally support remembering. -

COGNITIVE REHABILITATION in a Severely Brain-Injured PATIENT with Amnesia, TETRAPLEGIA and ANARTHRIA
J Rehabil Med 2009; 41: 393–396 CASE REPORT COMMUNICATING USING THE EYES WITHOUT REMEMBERING IT: COGNITIVE REHABILITATION IN A severely BRAIN-INJURED PATIENT WITH AMNESIA, TETRAPLEGIA AND ANARTHRIA Luigi Trojano, MD1,2, Pasquale Moretta, PsyD2 and Anna Estraneo, MD2 From the 1Neuropsychology Laboratory, Department of Psychology, Second University of Naples, Caserta and 2Salvatore Maugeri Foundation, IRCCS, Scientific Institute of Telese Terme (BN), Telese Terme, Italy We describe here a case of cognitive rehabilitation in a young LIS (4), but they nonetheless experience extreme difficulties patient with closed head injury, who had dense anterograde in communicating. For this reason patients with LIS can ac- amnesia and such disabling neurological defects (tetraplegia tively interact with the environment only by means of complex and anarthria) that the condition evoked some features of communicative systems, based for instance on brain-computer an incomplete locked-in syndrome. After a prolonged period interface devices (6). of no communicative possibility, the patient underwent a Patients with TBI with extremely severe motor disturbances specific training, based on principles of errorless learning, (tetraplegia and anarthria) face the same difficulties as patients with the aim of using a computerized eye-tracker system. with complete or incomplete LIS, but, in contrast to patients Although, due to memory disturbances, the patient always with LIS, they show the typical cognitive defects observed after denied ever having used the eye-tracker system, learned to a TBI. Therefore, any rehabilitative treatment in such patients use the computerized device and improved interaction with will be difficult and discouraging, even when it is aimed only the environment. -
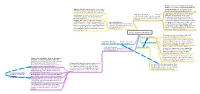
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Sustained Experience of Emotion After Loss of Memory in Patients with Amnesia
Sustained experience of emotion after loss of memory in patients with amnesia Justin S. Feinsteina,b,1, Melissa C. Duffa,c, and Daniel Tranela,b aDivision of Cognitive Neuroscience, Department of Neurology, University of Iowa College of Medicine, bDepartment of Psychology, and cDepartment of Communication Sciences and Disorders, University of Iowa, Iowa City, IA 52242-1053 Edited* by Marcus E. Raichle, Washington University, St. Louis, MO, and approved March 17, 2010 (received for review December 9, 2009) Can the experience of an emotion persist once the memory for what central feature of these patients’ condition is a profound induced the emotion has been forgotten? We capitalized on a rare impairment in forming new conscious memories about events that opportunity to study this question directly using a select group of unfold in their daily lives. This severe memory deficit confers a patients with severe amnesia following circumscribed bilateral unique opportunity to investigate whether the experience of an damage to the hippocampus. The amnesic patients underwent a emotion can outlast the memory for what caused it. sadness induction procedure (using affectively-laden film clips) to The dissociation between emotion and memory in patients with ascertain whether their experience of sadness would persist beyond amnesia harkens back to 1911, when the Swiss neurologist Cla- their memory for the sadness-inducing films. The experimentshowed parède concealed a pin between his fingers while greeting one of his that the patients continued to experience elevated levels of sadness amnesic patients with a handshake (18). The sharp pin surprised the well beyond the point in time at which they had lost factual memory patient and elicited a small amount of pain that quickly dissipated. -

Psych 102 Chapter 12 Presentation
7/26/19 Learning and Memory Chapter 12 Learning as the Storage of Memories Brain Changes in Learning Garrett: Brain & Behavior 4e Learning Deficiencies and Disorders 1 Learning as the Storage of Memories Figure 12.1: Temporal Lobe Structures Involved in Amnesia • Henry Molaison (HM) had frequent epileptic seizures • Treatment removed his hippocampal formation & amygdala. • *Anterograde amnesia: unable to form new memories. • *Retrograde amnesia: few memories decade before surgery • Amnesia usually affects declarative memory (dates, events, etc) Learning as the Storage of Memories Figure 12.2: Stages of Consolidation • *Consolidation: brain forms permanent representation of memory • Short-term (working) memory • Long-term memory • *Long-lasting memory – stored in cortical areas other than the hippocampus Learning as the Storage of Memories Figure 12.4: Hippocampal Activity Related to Consolidation Garrett: Brain & Behavior 4e 4 SOURCE: From “Hippocampal, But Not Amygdala Activity at Encoding Correlates With Long-Term Free Recall of Nonemotional Information,” by M. T. Alkire et al., 1998, PNAS, 95, pp. 14506–14510, fig. 1, lower left image, p. 14507. Learning as the Storage of Memories Figure 12.5: Human Hippocampal Activity During Retrieval • *Retrieval: Accessing stored memories. • Glutamate required for consolidation and retrieval. • *Blocking glutamate receptors prevents consolidation and retrieval • Prefrontal area: directs search strategy for retrieval in hippocampus Learning as the Storage of Memories Figure 12.6: Functional MRI Scans of Brains During Perception and Recall Garrett: Brain & Behavior 4e 6 Learning as the Storage of Memories Figure 12.7: Recordings from Place Cells in a Rat in a Circular Runway • Place cells • Increase firing when individual is in a specific location in an environment • Collectively form a “spatial map” • Dependent on environmental cues and landmarks • Also found in humans and primates SOURCE: From “Neural Plasticity in the Ageing Brain,” by S. -

Memory and Metamemory: a Study of the Feeling-Of-Knowing Phenomenon in Amnesic Patients
Journal of Experimental Psychology: Copyrighl 1986 by the American Psychological Association, Inc. Learning, Memory, and Cognition O278-7393/86/SO0.75 1986, Vol. 12, No. 3,452-460 Memory and Metamemory: A Study of the Feeling-of-Knowing Phenomenon in Amnesic Patients Arthur P. Shimamura and Larry R. Squire Veterans Administration Medical Center, San Diego and Department of Psychiatry, University of California, San Diego, School of Medicine Accuracy of the feeling of knowing was tested in patients with KorsakofTs syndrome, patients pre- scribed electroconvulsive therapy, four other cases of amnesia, and control subjects. In Experiment 1, we tested feeling-of-knowing accuracy for the answers to general information questions that could not be recalled. Subjects were asked to rank nonrecalled questions in terms of how likely they thought they would be able to recognize the answers and were then given a recognition test for these items. Only patients with KorsakofTs syndrome were impaired in making feeling-of-knowing predictions. The other amnesic patients were as accurate as control subjects in their feeling-of-knowing predic- tions. In Experiment 2, we replicated these findings in a sentence memory paradigm that tested newly learned information. The results showed that impaired metamemory is not an obligatory feature of amnesia, because amnesia can occur without detectable metamemory deficits. The im- paired metamemory exhibited by patients with KorsakofTs syndrome reflects a cognitive impair- ment that is not typically observed in other forms of amnesia. Often one experiences a sense or feeling of knowing some effects (Diamond & Rozin, 1984; Graf, Squire, & Mandler, information without being able to recall it. -

The Relationship Between Anterograde and Retrograde Amnesia in Alcoholic Wernicke-Korsakoff Syndrome1
Psychological Medicine, 1991, 21, 11-14 Printed in Great Britain EDITORIAL The relationship between anterograde and retrograde amnesia in alcoholic Wernicke-Korsakoff Syndrome1 The Wernicke-Korsakoff Syndrome (WKS) can be described as a constellation of neuro- psychological deficits the most prominent of which is a profound amnesic syndrome. The neurological impairment underlying WKS is directly attributable to thiamine deficiency, which has the consequential effect of Wernicke's encephalopathy, resulting in vascular lesions of the midline diencephalic nuclei (Victor et al. 1989). The most common aetiology of this thiamine deficiency is chronic alcoholism, although it must be emphasized that the same form of neurological impairment can arise in other forms of nutritional disorder (e.g. Becker el al. 1990). WKS patients exhibit both a severe anterograde amnesia, manifest by poor performance on memory tasks such as free recall and recognition, and retrograde amnesia, as shown by an inability to recall or recognize information relating to the pre-morbid period. Reviews of WKS indicate that the co-occurrence of anterograde and retrograde amnesia is a persistent feature of the syndrome (Parkin & Leng, 1988). Indeed, to my knowledge, there is no well-described instance of WKS in which this relationship has not been reported. This regular state of affairs leads directly to the possibility that the anterograde and retrograde deficits may have a common origin. The consistent association of WKS with chronic alcoholism has been invoked in attempts to provide a single explanation for the anterograde and retrograde deficits associated with WKS. This view, which has been termed the continuity hypothesis (e.g. -

Three Cases of Enduring Memory Impairment After Bilateral Damage Limited to the Hippocampal Formation
The Journal of Neuroscience, August 15, 1996, 16(16):5233–5255 Three Cases of Enduring Memory Impairment after Bilateral Damage Limited to the Hippocampal Formation Nancy L. Rempel-Clower,3 Stuart M. Zola,1,2,3 Larry R. Squire,1,2,3 and David G. Amaral4 1Veterans Affairs Medical Center, San Diego, California 92161, Departments of 2Psychiatry and 3Neurosciences, University of California at San Diego, La Jolla, California 92093, and 4Department of Psychiatry and Center for Neuroscience, University of California at Davis, Davis, California 95616 Patient RB (Human amnesia and the medial temporal region: anterograde memory impairment. (2) Bilateral damage beyond enduring memory impairment following a bilaterial lesion limited the CA1 region, but still limited to the hippocampal formation, to field CA1 of the hippocampus, S. Zola-Morgan, L. R. Squire, can produce more severe anterograde memory impairment. (3) and D. G. Amaral, 1986, J Neurosci 6:2950–2967) was the first Extensive, temporally graded retrograde amnesia covering 15 reported case of human amnesia in which detailed neuropsy- years or more can occur after damage limited to the hippo- chological analyses and detailed postmortem neuropathologi- campal formation. Findings from studies with experimental an- cal analyses demonstrated that damage limited to the hip- imals are consistent with the findings from amnesic patients. pocampal formation was sufficient to produce anterograde The present results substantiate the idea that severity of mem- memory impairment. Neuropsychological and postmortem ory impairment is dependent on locus and extent of damage neuropathological findings are described here for three addi- within the hippocampal formation and that damage to the tional amnesic patients with bilateral damage limited to the hippocampal formation can cause temporally graded retro- hippocampal formation. -

Acute Korsakoff-Like Amnestic Syndrome Resulting from Left
Acute Korsakoff-Like Amnestic Syndrome CASE REPORT Resulting from Left Thalamic Infarction Following R. Rahme a Right Hippocampal Hemorrhage R. Moussa SUMMARY: Korsakoff-like amnestic syndromes have been rarely described following structural le- A. Awada sions of the central nervous system. In this report, we describe a case of acute Korsakoff-like I. Ibrahim syndrome resulting from the combination of a left anteromedian thalamic infarct and a right hippocam- Y. Ali pal hemorrhage. We also review the literature relevant to the neuropathology and pathophysiology of J. Maarrawi Korsakoff syndrome and anterograde amnesia. T. Rizk G. Nohra N. Okais E. Samaha orsakoff syndrome has been classically attributed to thiamine Kdeficiency from chronic alcoholism or severe malnutrition.1 However, Korsakoff-like syndromes have been occasionally re- ported following focal structural lesions of the central nervous system.2-6 We present a case of Korsakoff-like amnestic syn- drome resulting from the combination of a left anteromedian thalamic infarct and a right hippocampal hemorrhage. Case Report A 63-year-old right-handed nonalcoholic diabetic male patient pre- sented for sudden-onset headache with vomiting. Findings of a neu- rologic examination were unremarkable. CT (Fig 1A), and MR imag- BRAIN ing (Fig 1B) of the brain revealed a localized hemorrhage in the right hippocampal formation extending into the temporal and occipital horns of the lateral ventricle and the deep Sylvian fissure. The patient was admitted to the hospital and managed conservatively. He re- mained stable until 2 weeks later when he suddenly developed confu- CASE REPORT sion. At that time, he was found to be awake and alert but disoriented as to time and place. -

Retrograde Amnesia and Remote Memory Impairment
Neuropsychobgu. Vol. 19, No 3, pp. 337-356, 1981. 0028~3932/81/030337-1902.00/0 Prmted in Great Butam. c 1981 Pergamon Press Ltd RETROGRADE AMNESIA AND REMOTE MEMORY IMPAIRMENT NEAL J. COHEN* Department of Neurosciences, University of California School of Medicine, La Jolla, California, U.S.A and LARRY R. SQUIRE? Veterans Administration Medical Center, San Diego, California and the Department of Psychiatry, University of California School of Medicine, La Jolla, California, U.S.A. (Receired 3 Noormher 1980) Abstract--The status of remote memory in case N.A., patients receiving bilateral ECT, and patients with KorsakoBsyndrome was assessed using seven different tests. The extent and pattern of remote memory impairment depended upon the etiology of amnesia, appearing as a relatively brief retrograde amnesia for N.A. and patients receiving ECT and as an extensive impairment covering many decades for patients with KorsakoB syndrome. Differences in remote memory impairment could not be explained by differences in the severity ofanterograde amnesia, indicating that amnesia is not a unitary disorder. We suggest that brief retrograde amnesia is typically present whenever amnesia occurs and that extensive remote memory impairment is a distinct entity related to superimposed cognitive deficits. These ideas and a review of previous studies lead to a comprehensive proposal for understanding retrograde amnesia and remote memory impairment. THE AMNESIC syndrome has been the subject of much interest in neuropsychology because of what it might reveal about the organization and neurological foundations of normal memory. The phenomenon of retrograde amnesia, that loss of memory can occur for the period before the precipitating incident, has had a particularly large impact on ideas about normal memory function [l-5]. -

Transient Global Amnesia
Acta Biomed 2014; Vol. 85, N. 3: 229-235 © Mattioli 1885 Focus on Transient global amnesia Chiara Marazzi1, Umberto Scoditti2, Andrea Ticinesi3-4, Antonio Nouvenne3-4, Federica Pigna1, Loredana Guida4, Ilaria Morelli4, Loris Borghi3, Tiziana Meschi3-4 1 Post-Graduate School of Emergency-Urgency Medicine, University of Parma, Parma, Italy; 2 Stroke Unit and Neurology Clinic, University Hospital of Parma, Parma, Italy; 3 Department of Clinical and Experimental Medicine, University of Parma, Parma, Italy; 4 Internal Medicine and Critical Subacute Care Unit, University Hospital of Parma, Parma, Italy Summary. Transient Global Amnesia (TGA) is a clinical syndrome characterized by temporary inability to form new memories described as anterograde amnesia. It is associated with retrograde amnesia and repetitive questioning. During the attack patients remain conscious and communicative and personal identity is pre- served. Focal neurological symptoms and epileptic features are absent and general conditions appear intact. The ability to store new memories gradually recovers and subjects return to normal conditions except for a substantial amnestic gap for the duration of the attack. TGA has an incidence of 3-8 per 100 000 people per year. It usually affects patients between the ages of 50 and 70 years, at an average age of 61 years; occurrence in patients younger than 40 years of age is rare. The rate of recurrence is between 6% and 10% per years. No gender prevalence has been recorded. The patients with definite TGA have a very good prognosis; their rate of subsequent major vascular events is less than 1% per year. Key words: transient global amnesia, episodic memory, hippocampus.