Three Cases of Enduring Memory Impairment After Bilateral Damage Limited to the Hippocampal Formation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Memory Part 1: Overview
FUNCTIONAL VIGNETTE Memory Part 1: Overview F.D. Raslau, A.P. Klein, J.L. Ulmer, V. Mathews, and L.P. Mark common layman’s conception of memory is the simple stor- Aage and retrieval of learned information that often evokes comparison to a filing system. Our everyday experience of mem- ory, however, is in fact a complicated multifactorial process that consists of both conscious and unconscious components, and de- pends on the integration of a variety of information from distinct functional systems, each processing different types of information by different areas of the brain (substrates), while working in a concerted fashion.1-4 In other words, memory is not a singular process. It represents an integrated network of neurologic tasks and connections. In this light, memory may evoke comparison with an orchestra composed of many different instruments, each making different sounds and responsible for different parts of the score, but when played together in the proper coordinated fash- ion, making an integrated musical experience that is greater than the simple sum of the individual instruments. Memory consists of 2 broad categories (Fig 1): short- and long-term memory.5 Short-term memory is also called work- ing memory. Long-term memory can be further divided into FIG 1. Classification of memory. declarative and nondeclarative memory. These are also re- ferred to as explicit/conscious and implicit/unconscious mem- heard (priming), and salivating when you see a favorite food ory, respectively. Declarative or explicit memory consists of (conditioning). episodic (events) and semantic (facts) memory. Nondeclara- The process of memory is dynamic with continual change over tive or implicit memory consists of priming, skill learning, and time.5 Memory traces are initially formed as a series of connections conditioning. -

DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? the Role of Explicit Memory and Attention in Attitude Change Matthew D
PSYCHOLOGICAL SCIENCE Research Article DO AMNESICS EXHIBIT COGNITIVE DISSONANCE REDUCTION? The Role of Explicit Memory and Attention in Attitude Change Matthew D. Lieberman, Kevin N. Ochsner, Daniel T. Gilbert, and Daniel L. Schacter Harvard University Abstract—In two studies, we investigated the roles of explicit mem- CONSCIOUS CONTENT: THE ROLE OF ory and attentional resources in the process of behavior-induced atti- EXPLICIT MEMORY tude change. Although most theories of attitude change (cognitive Explicit memory refers to one’s ability to consciously recollect dissonance and self-perception theories) assume an important role for past events, behaviors, and experiences (Schacter, Chiu, & Ochsner, both mechanisms, we propose that behavior-induced attitude change 1993) and thus is a central component of most social abilities. Indeed, can be a relatively automatic process that does not require explicit people’s memory for the identities and actions of other people and memory for, or consciously controlled processing of, the discrepancy themselves forms the adhesive that gives them a continuing sense of between attitude and behavior. Using a free-choice paradigm, we place in their social world. Revising personal attitudes and beliefs in found that both amnesics and normal participants under cognitive response to a counterattitudinal behavior naturally seems to depend on load showed as much attitude change as did control participants. retrospective capacities. If Aesop’s forlorn lover of grapes could not remember that the grapes were out of reach, he would have no reason A fox saw some ripe black grapes hanging from a trellised vine. He resorted to to persuade himself of their reduced quality. -

Mild Cognitive Impairment Douglas W
Mild Cognitive Normal Impairment (MCI) Mild Cognitive Impairment Douglas W. Scharre, MD Director, Division of Cognitive Neurology Ohio State University Dementia Dementia Definition • Syndrome of acquired persistent intellectual impairment • Persistent deficits in at least three of the following: Definitions 9 Memory 9 Language 9 Visuospatial 9 Personality or emotional state 9 Cognition • Resulting in impairment in Activities of Daily Living (ADL) 1 Mild Cognitive Impairment MCI Related (MCI) Definition Conditions • Definitions vary - be careful! AAMI: Age-Associated Memory Impairment • Petersen criteria • Non-disease, aging-related decline in – Memory complaint memory – Memory loss on testing greater that 1.5 standard • Diagnostic criteria are ambiguous deviations below normal for age • Complaints of memory loss more related – Other non-memory cognitive domains normal or to affect/personality than test scores mild deficits (language, visuospatial, executive, personality or emotional state) • Prevalence in the elderly varies from 18% up to 38% depending on the study – Mostly normal activities of daily living Barker et al. Br J Psychiatry 1995;167:642-8 Arch Neurol 1999;56:303-8 Hanninen et al. Age and Aging 1996;25:201-5 MCI Related MCI Related Conditions Conditions • Age-Associated Memory Impairment Benign Senescent Forgetfulness or Mild (AAMI) Memory Impairment (MMI) • Memory tests > 2 s.d. below normal age and • Benign Senile Forgetfulness or Mild education matched individuals Memory Impairment (MMI) • Normal non-memory cognitive domains -

The Three Amnesias
The Three Amnesias Russell M. Bauer, Ph.D. Department of Clinical and Health Psychology College of Public Health and Health Professions Evelyn F. and William L. McKnight Brain Institute University of Florida PO Box 100165 HSC Gainesville, FL 32610-0165 USA Bauer, R.M. (in press). The Three Amnesias. In J. Morgan and J.E. Ricker (Eds.), Textbook of Clinical Neuropsychology. Philadelphia: Taylor & Francis/Psychology Press. The Three Amnesias - 2 During the past five decades, our understanding of memory and its disorders has increased dramatically. In 1950, very little was known about the localization of brain lesions causing amnesia. Despite a few clues in earlier literature, it came as a complete surprise in the early 1950’s that bilateral medial temporal resection caused amnesia. The importance of the thalamus in memory was hardly suspected until the 1970’s and the basal forebrain was an area virtually unknown to clinicians before the 1980’s. An animal model of the amnesic syndrome was not developed until the 1970’s. The famous case of Henry M. (H.M.), published by Scoville and Milner (1957), marked the beginning of what has been called the “golden age of memory”. Since that time, experimental analyses of amnesic patients, coupled with meticulous clinical description, pathological analysis, and, more recently, structural and functional imaging, has led to a clearer understanding of the nature and characteristics of the human amnesic syndrome. The amnesic syndrome does not affect all kinds of memory, and, conversely, memory disordered patients without full-blown amnesia (e.g., patients with frontal lesions) may have impairment in those cognitive processes that normally support remembering. -

COGNITIVE REHABILITATION in a Severely Brain-Injured PATIENT with Amnesia, TETRAPLEGIA and ANARTHRIA
J Rehabil Med 2009; 41: 393–396 CASE REPORT COMMUNICATING USING THE EYES WITHOUT REMEMBERING IT: COGNITIVE REHABILITATION IN A severely BRAIN-INJURED PATIENT WITH AMNESIA, TETRAPLEGIA AND ANARTHRIA Luigi Trojano, MD1,2, Pasquale Moretta, PsyD2 and Anna Estraneo, MD2 From the 1Neuropsychology Laboratory, Department of Psychology, Second University of Naples, Caserta and 2Salvatore Maugeri Foundation, IRCCS, Scientific Institute of Telese Terme (BN), Telese Terme, Italy We describe here a case of cognitive rehabilitation in a young LIS (4), but they nonetheless experience extreme difficulties patient with closed head injury, who had dense anterograde in communicating. For this reason patients with LIS can ac- amnesia and such disabling neurological defects (tetraplegia tively interact with the environment only by means of complex and anarthria) that the condition evoked some features of communicative systems, based for instance on brain-computer an incomplete locked-in syndrome. After a prolonged period interface devices (6). of no communicative possibility, the patient underwent a Patients with TBI with extremely severe motor disturbances specific training, based on principles of errorless learning, (tetraplegia and anarthria) face the same difficulties as patients with the aim of using a computerized eye-tracker system. with complete or incomplete LIS, but, in contrast to patients Although, due to memory disturbances, the patient always with LIS, they show the typical cognitive defects observed after denied ever having used the eye-tracker system, learned to a TBI. Therefore, any rehabilitative treatment in such patients use the computerized device and improved interaction with will be difficult and discouraging, even when it is aimed only the environment. -
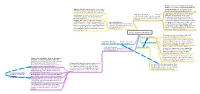
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Sustained Experience of Emotion After Loss of Memory in Patients with Amnesia
Sustained experience of emotion after loss of memory in patients with amnesia Justin S. Feinsteina,b,1, Melissa C. Duffa,c, and Daniel Tranela,b aDivision of Cognitive Neuroscience, Department of Neurology, University of Iowa College of Medicine, bDepartment of Psychology, and cDepartment of Communication Sciences and Disorders, University of Iowa, Iowa City, IA 52242-1053 Edited* by Marcus E. Raichle, Washington University, St. Louis, MO, and approved March 17, 2010 (received for review December 9, 2009) Can the experience of an emotion persist once the memory for what central feature of these patients’ condition is a profound induced the emotion has been forgotten? We capitalized on a rare impairment in forming new conscious memories about events that opportunity to study this question directly using a select group of unfold in their daily lives. This severe memory deficit confers a patients with severe amnesia following circumscribed bilateral unique opportunity to investigate whether the experience of an damage to the hippocampus. The amnesic patients underwent a emotion can outlast the memory for what caused it. sadness induction procedure (using affectively-laden film clips) to The dissociation between emotion and memory in patients with ascertain whether their experience of sadness would persist beyond amnesia harkens back to 1911, when the Swiss neurologist Cla- their memory for the sadness-inducing films. The experimentshowed parède concealed a pin between his fingers while greeting one of his that the patients continued to experience elevated levels of sadness amnesic patients with a handshake (18). The sharp pin surprised the well beyond the point in time at which they had lost factual memory patient and elicited a small amount of pain that quickly dissipated. -

POST TRAUMATIC AMNESIA SCREENING and MANAGEMENT GUIDELINE Trauma Service Guidelines Title: Post Traumatic Amnesia Screening and Management Developed By: K
TRM 01.01 POST TRAUMATIC AMNESIA SCREENING AND MANAGEMENT GUIDELINE Trauma Service Guidelines Title: Post Traumatic Amnesia Screening and Management Developed by: K. Gumm, T, Taylor, K, Orbons, L, Carey, PTA Working Party Created: Version 1.0, April 2007 Revised by: K. Liersch, K. Gumm, E. Hayes, E. Thompson, K. Henderson & Advisory Committee on Trauma Revised: V 5.0 Jun 2020, V 4.0 Oct 2017, V3.0 Mar 2014, V2.0 Apr 2011 Table of Contents What is Post Traumatic Amnesia (PTA)?........................................................................................................................................................1 Screening Criteria for PTA..............................................................................................................................................................................5 Abbreviated Assessment of MBI using the Abbreviated Westmead PTA Scale (A-WPTAS) ..........................................................................5 Discharging a Patient Home with a Mild Brain Injury ....................................................................................................................................6 Moderate and Severe Brain Injury.................................................................................................................................................................7 Assessment of Moderate and Severe Brain Injury using the Westmead PTA Scale (Westmead)..................................................................7 Management of the patient suffering -

Partners Healthcare: Clinical Pathway for Imaging in Mild Traumatic Brain Injury
Partners Healthcare: Clinical Pathway for Imaging in Mild Traumatic Brain Injury Please answer the following 3 questions, selecting all of the responses that apply: QUESTION 1: Does the patient have mild traumatic brain injury (MTBI)? Injury to the head, resulting from blunt trauma or acceleration or deceleration forces, AND, ONE OR MORE OF THE FOLLOWING conditions attributable to the head injury: Transient confusion, disorientation, or impaired consciousness Dysfunction of memory (amnesia) around the time of injury Observed signs of other neurologic or neuropsychological dysfunction Loss of consciousness lasting 30 minutes or less QUESTION 2: Is the patient eligible for the clinical pathway? Exclusion Criteria: The patient must have NONE of the following: Penetrating head trauma GCS score < 15 on initial evaluation Age < 16 years Presenting to ED more than 24‐hours after injury Severe Multisystem Trauma, defined as: Hemodynamic instability (HR > 120, SBP <90) Major trauma with suspected or proven life‐threatening injuries QUESTION 3: Did the patient have loss of consciousness (LOC) or post‐traumatic amnesia? YES NO Head CT is unlikely to be helpful UNLESS the Head CT is unlikely to be helpful UNLESS the patient has one or more of the following: patient has one or more of the following: Focal neurologic deficit Focal neurologic deficit Coagulopathy Coagulopathy Vomiting Vomiting GCS score < 15 GCS score < 15 Age > 60 years Age 65 years Headache Severe headache Physical evidence of trauma above the clavicles -

Memory Dysfunction INTRODUCTION Distributed Brain Networks
Memory Dysfunction REVIEW ARTICLE 07/09/2018 on SruuCyaLiGD/095xRqJ2PzgDYuM98ZB494KP9rwScvIkQrYai2aioRZDTyulujJ/fqPksscQKqke3QAnIva1ZqwEKekuwNqyUWcnSLnClNQLfnPrUdnEcDXOJLeG3sr/HuiNevTSNcdMFp1i4FoTX9EXYGXm/fCfl4vTgtAk5QA/xTymSTD9kwHmmkNHlYfO by https://journals.lww.com/continuum from Downloaded By G. Peter Gliebus, MD Downloaded CONTINUUM AUDIO INTERVIEW AVAILABLE ONLINE from https://journals.lww.com/continuum ABSTRACT PURPOSE OF REVIEW: This article reviews the current understanding of memory system anatomy and physiology, as well as relevant evaluation methods and pathologic processes. by SruuCyaLiGD/095xRqJ2PzgDYuM98ZB494KP9rwScvIkQrYai2aioRZDTyulujJ/fqPksscQKqke3QAnIva1ZqwEKekuwNqyUWcnSLnClNQLfnPrUdnEcDXOJLeG3sr/HuiNevTSNcdMFp1i4FoTX9EXYGXm/fCfl4vTgtAk5QA/xTymSTD9kwHmmkNHlYfO RECENT FINDINGS: Our understanding of memory formation advances each year. Successful episodic memory formation depends not only on intact medial temporal lobe structures but also on well-orchestrated interactions with other large-scale brain networks that support executive and semantic processing functions. Recent discoveries of cognitive control networks have helped in understanding the interaction between memory systems and executive systems. These interactions allow access to past experiences and enable comparisons between past experiences and external and internal information. The semantic memory system is less clearly defined anatomically. Anterior, lateral, and inferior temporal lobe regions appear to play a crucial role in the function of the semantic -

Psych 102 Chapter 12 Presentation
7/26/19 Learning and Memory Chapter 12 Learning as the Storage of Memories Brain Changes in Learning Garrett: Brain & Behavior 4e Learning Deficiencies and Disorders 1 Learning as the Storage of Memories Figure 12.1: Temporal Lobe Structures Involved in Amnesia • Henry Molaison (HM) had frequent epileptic seizures • Treatment removed his hippocampal formation & amygdala. • *Anterograde amnesia: unable to form new memories. • *Retrograde amnesia: few memories decade before surgery • Amnesia usually affects declarative memory (dates, events, etc) Learning as the Storage of Memories Figure 12.2: Stages of Consolidation • *Consolidation: brain forms permanent representation of memory • Short-term (working) memory • Long-term memory • *Long-lasting memory – stored in cortical areas other than the hippocampus Learning as the Storage of Memories Figure 12.4: Hippocampal Activity Related to Consolidation Garrett: Brain & Behavior 4e 4 SOURCE: From “Hippocampal, But Not Amygdala Activity at Encoding Correlates With Long-Term Free Recall of Nonemotional Information,” by M. T. Alkire et al., 1998, PNAS, 95, pp. 14506–14510, fig. 1, lower left image, p. 14507. Learning as the Storage of Memories Figure 12.5: Human Hippocampal Activity During Retrieval • *Retrieval: Accessing stored memories. • Glutamate required for consolidation and retrieval. • *Blocking glutamate receptors prevents consolidation and retrieval • Prefrontal area: directs search strategy for retrieval in hippocampus Learning as the Storage of Memories Figure 12.6: Functional MRI Scans of Brains During Perception and Recall Garrett: Brain & Behavior 4e 6 Learning as the Storage of Memories Figure 12.7: Recordings from Place Cells in a Rat in a Circular Runway • Place cells • Increase firing when individual is in a specific location in an environment • Collectively form a “spatial map” • Dependent on environmental cues and landmarks • Also found in humans and primates SOURCE: From “Neural Plasticity in the Ageing Brain,” by S. -

Consolidation Theory and Retrograde Amnesia in Humans
Psychonomic Bulletin & Review 2002, 9 (3), 403-425 THEORETICAL AND REVIEW ARTICLES Consolidation theory and retrograde amnesia in humans ALAN S. BROWN Southern Methodist University, Dallas, Texas Recent researchon the cognitive dysfunctions experienced by human anmesic patients indicates that very long term (multidecade) changes may occur in memory. Flat retrograde amnesia (RA), consisting of a uniform memory deficit for information from all preamnesia time periods, indicates a simple, mono- lithic retrievalproblem, whereas graded RA, with greater memory deficits for information from recent as opposed to remote time periods, suggests the presence of a gradual long-term encoding, or consol- idation, process. An evaluation of 247 outcomes from 61 articlesprovides strong evidence of graded RA across different cerebral injuries, materials, and test procedures, as well as in measures of both ab- solute and relative(patientvs. control) performance.Future conceptualizationsof human memory should address the possibility that memories increase in resistanceto forgetting,or reduction in trace fragility, across many decades. The concept of consolidation was introduced over a A major problem with integrating the concept of con- centuryago by Müller and Pilzecker(1900; see also Burn- solidationinto mainstream cognitivepsychologyis the dif- ham, 1903).While Müller and Pilzecker (1900) originally ficulty of a direct empirical test in humans. Whereas ani- conceivedof consolidationoccurringovershort periods of mal research has repeatedlyevaluatedconsolidationtheory