Magenta and CI Basic Red 9 Are of Briliant Hue, Exhibit High Tinctorial Strength, Are Relatively Inexpensive and May Be Applied to a Wide Range of Substrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21
Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21 भारत सरकार Government of India स्वास्थ्य सेवा महानिदेशालय Directorate General of Health Services स्वास्थ्य एवं पररवार कल्याण मंत्रालय Ministry of Health & Family Welfare ग मेनडकल कॉलेज एवं श्रीमती सुचेता कृपलािी अस्पतालﴂलेडी हनड Lady Hardinge Medical College & Smt. Sucheta Kriplani Hospital शहीद भगत नसंह मागग, िई नदल्ली – ११०००१ Shaheed Bhagat Singh Marg, New Delhi-110001 ३ नसतम्बर २०२० / 3rd September 2020 भंडार अिुभाग/Stores Section Tender Documents for Advertised Tender Enquiry for running rate contract of Kits, Chemicals & Reagents required for Lady Hardinge Medical College & Associated Hospitals New Delhi (Two Bid System) Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21 Dated: 3rd September 2020 Amount of Bid Security: Rs. 2,00,000.00 (Rs. Two Lakh Only) Tender Fee: Rs. 0 (Can be downloaded from Central Public Procurement Portal or LHMC Website) CRITICAL DATES Start Date of Sale of Tender: 04/09/2020 11.00 AM to 1.30 PM and from 2.30 PM to 4.00 PM End Date of Sale of Tender: 12/10/2020 4.00 PM Start Date for Submission of Tender: 13/10/2020, 10.00 AM onwards, Time Schedule for Submission of Tender: 14/10/2020, upto 11.00 AM Time Schedule for Opening of Tender: 14/10/2020, 11.30 AM A. INSTRUCTIONS 1. LHMC & Associated Hospitals proposed to enter into a rate-contract (R/C) for the supply of Chemicals, Reagents & Kits valid for a period of 24 months from the date of opening of the Price Bid, can be extended for a period of 12 months or more on mutual consent on existing terms & conditions. -

A Study of Rawitz's 'Inversion Staining' by ALEKSANDRA PRZEL^CKA
231 A Study of Rawitz's 'Inversion Staining' By ALEKSANDRA PRZEL^CKA {From the Cytological Laboratory, Department of Zoology, University Museum, Oxford, and the Nencki Institute, 3 Pasteur St., Warsaw 22; present address, Nencki Institute) SUMMAHY The Rawitz method involves mordanting with tannic acid and potassium antimony tartrate, and staining with basic fuchsine. The mordanting causes basic fuchsine to act as though it were an acid dye ('inversion staining'). A modification of the method is described in the present paper. This modification makes it possible to obtain the same results in a shorter time. The chief substances stained by Rawitz's method are phospholipids, certain pro- teins, and certain polysaccharides. Although the method cannot be regarded as a cytochemical test in the strict sense, yet it gives useful indications of chemical composition and in addition is valuable to the morphological cytologist as a technique for showing certain cytoplasmic inclusions (mitotic spindle, acrosome, mitochondria, 'Golgi apparatus' of certain cells). INTRODUCTION T is well known that the so-called 'Golgi apparatus' is extremely difficult to I reveal by any staining method. Baker, in the course of his investigation on this organelle in the epididymis of the mouse, found that it can be stained by basic fuchsin after a special mordanting process (1957). The method was taken from Rawitz (1895), who found that basic fuchsin, if mordanted with tannic acid and potassium antimony tartrate, loses the character of a dye for chro- matin and colours the cytoplasm instead. Rawitz called this effect 'inversion staining'. Since this technique, when applied to various kinds of cytological material, gave good selectivity in visualizing certain delicate cell structures, it seemed interesting to investigate the nature of the chemical compounds which are responsible for positive Rawitz staining. -

Revisions Inserts Rev from Rev to JOB
BALTSO0191 Version 11.0 Template 4 Revisions Inserts Rev from Rev to JOB # 06 07 52-17 Notes: 1. BD Catalog Number: 212525, 212526, 212527, 212528, 212531, 212532, 212539, 212542, 212543, 212544, 212545 2. Blank (Sheet) Size: Length: 25.5” Width: 22” 3. Number of Pages: 28 Number of Sheets: 1 4. Page Size: Length: 8.5” Width: 5.5” Final Folded Size: 4.25” x 5.5” 5. Ink Colors: No. of Colors: 2 PMS#: 032 Red; Standard Black 6. Printed two sides: Yes X No 7. Style (see illustrations below): # 5 W W W W W W W 8. Vendor Printed X Online/In House Printed Web 9. See specication control no. N/A for material information. 10. Graphics are approved by Becton, Dickinson and Company. Supplier has the responsibility for using the most current approved revision level. Label Design COMPANY CONFIDENTIAL. THIS DOCUMENT IS THE PROPERTY OF BECTON, DICKINSON AND Becton, Dickinson and Company Proofer COMPANY AND IS NOT TO BE USED OUTSIDE THE COMPANY WITHOUT WRITTEN PERMISSION. 7 Loveton Circle Sparks, MD 21152 USA Checked By Category and Description Sheet: 1 of 29 Part Number: Package Insert, 8820191JAA Gram Stain Kits and Reagents Scale: N/A A B Gram Stain Kits and Reagents English: pages 1 – 5 Italiano: pagine 14 – 18 8820191JAA(07) Français : pages 5 – 9 Español: páginas 19 – 23 2017-09 Deutsch: Seiten 10 – 14 Contact your local BD representative for instructions. / Свържете се с местния представител на BD за инструкзии. / Pokyny vám poskytne místní zástupce společnosti BD. / Kontakt den lokale BD repræsentant for at få instruktioner. -

Factors Affecting the Adsorption of Some Ionic Dyes on the Surface of Modify Cao from Eggshell
Asian Journal of Applied Sciences (ISSN: 2321 – 0893) Volume 07 – Issue 01, February 2019 Factors Affecting the Adsorption of Some Ionic Dyes on the Surface of Modify CaO from Eggshell Ibtighaa K. Radhi, Mouayed A. Hussein, Zaki N. Kadhim* Department of Chemistry, College of Science, University of Basrah Basrah, Iraq *Corresponding author’s emails: zekinasser99 [AT] yahoo.com ________________________________________________________________________________________________ ABSTRACT--- In this paper, calcium oxide (CaO) was produced by the thermal treatment of eggshell. The doping process with silver iodide (AgI), oxygen (O), sulfur(S) and nitrogen (N) was achieved by adsorbents. The adsorption of Acid fuchsine (AF), Indigo Carmine (IC), Nigrosine (NG) and Alizarine Red S (AR) on the surface of these particles was studied. The different conditions affecting the adsorption process, such as the time of equilibrium, the primary concentration of the studied dyes, the amount of the adsorbent, the acidic function, the speed of the pruning motion and the temperature were studied. The pH stability time (5-10 minutes), IC and NG (30 minutes) and AR were (90 minutes). The effect of temperature was also studied within the range (25-45 ° C). The results showed that the adsorption capacity increased by increasing the temperature, ie the reaction is endothermic. The study showed the effect of the acidic function on the percentage of pigmentation. The percentage was increased by increasing the acidic function in the basal circles on the surfaces except for the AR dye. It decreased the percentage by increasing the acidic function. The effect of the weight of the adsorbent was studied on the percentage of adsorption. -

Pituitary Gland
Part 6: Pituitary Gland Normal Physiology and Structure The pituitary gland comprises the adenohypophysis, which is made up of the pars distalis, pars intermedia and pars tuberalis and the neurohypophysis which includes the pars nervosa, infundibular stem and median eminence. The pars distalis forms the largest proportion of the gland and functions as the overall regulator of peripheral endocrine function by synthesizing and secreting at least 6 major trophic hormones. These include growth hormone (GH), prolactin (PrL), adrenocorticotrophic hormone (ACTH), thyroid stimulating hormone (TSH), luteinizing hormone (LH) and follicle stimulating hormone (FSH). Since this is the important area of the pituitary with respect to detecting endocrine active compounds, the rest of this section will concentrate only on this part of the pituitary. For reviews see (Page, 1994; Tucker, 1999; Greaves, 2007). Each hormone of the pars distalis is generally secreted by a seperate cell type, but some cells are able to secrete two hormones. The different hormones impart different staining properties to the cells. Using histological stains based on Orange G and periodic acid-Schiff (PAS), the cells of the pars distalis have been divided into acidophils (orange G positive), basophils (PAS positive) and chromophobes (absence of staining). In the rat, these have been reported to constitute 40, 10 and 50% respectively of the cell population of the pars distalis. The staining characteristics are dependent on the level of secretory activity, and when the cells have just secreted their granules or when secretory activity is increased, all the cells take on chromophobic characteristics due to the relative abundance of secretory organelles (endoplasmic reticulum and Golgi) and relative lack of secretory granules. -

New Tetrachromic VOF Stain (Type III-G.S) for Normal and Pathological Fish Tissues C
ORIGINAL PAPER New Tetrachromic VOF Stain (Type III-G.S) for Normal and Pathological Fish Tissues C. Sarasquete,* M. Gutiérrez Instituto de Ciencias Marinas de Andalucía, CSIC Polígono Río San Pedro, Apdo oficial, Puerto Real, Cádiz, Spain richrome methods invariably use dyes in acid ©2005, European Journal of Histochemistry pH solvents, usually diluted in aqueous acetic Tacid, and the concentration of this acid A new VOF Type III-G.S stain was applied to histological sec- matches the concentration of dye. Staining depends tions of different organs and tissues of healthy and pathologi- largely on the attachment of dyes to proteins. The cal larvae, juvenile and adult fish species (Solea senegalensis; acid pH itself is necessary to maximise the amount Sparus aurata; Diplodus sargo; Pagrus auriga; Argyrosomus regius and Halobatrachus didactylus). In comparison to the of dye that will attach to tissue amino groups. original Gutiérrez´VOF stain, more acid dyes of contrasting Proteins have both positively (amino groups) and colours and polychromatic/metachromatic properties were negatively (carboxyl and hydroxyl) charged groups. incorporated as essential constituents of the tetrachromic VOF Usually one predominates and this will have an stain. This facilitates the selective staining of different basic tissues and improves the morphological analysis of histo- overall negative or positive charge (being an acid or chemical approaches of the cell components. The VOF-Type III a basic protein). These charges can, however, bal- G.S stain is composed of a mixture of several dyes of varying ance each other out to some degree. Phosphate size and molecular weight (Orange G< acid Fuchsin< Light green<Methyl Blue<Fast Green), which are used simultane- groups of DNA and binding-proteins are important ously, and it enables the individual tissues to be selectively dif- in nuclear staining.The ionisation of basic groups of ferentiated and stained. -

The Histochemical Distribution of Placental Calcium and Alkaline Phosphatase Activity Following Fetoplacental Dissociation in Th
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1976 The Histochemical Distribution of Placental Calcium and Alkaline Phosphatase Activity Following Fetoplacental Dissociation in the Albino Rat eric sigmond Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Medical Anatomy Commons Recommended Citation sigmond, eric, "The Histochemical Distribution of Placental Calcium and Alkaline Phosphatase Activity Following Fetoplacental Dissociation in the Albino Rat" (1976). Master's Theses. 2872. https://ecommons.luc.edu/luc_theses/2872 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1976 eric sigmond THE HISTOCHEMICAL DISTRIBUTION OF PLACENTAL CALCIUM AND ALKALINE PHOSPHATASE ACTIVITY FOLLOWING FETOPLACENTAL DISSOCIATION IN THE ALBINO RAT by Eric Sigmond A Thesis Submitted to the Faculty of the Graduate School of Loyola University of Chicago in Partial Fulfillment of the Requirements for the Degree of Master of Science February 1976 ACKNOWLEDGEMENT I wish to express my gratitude to Dr. Leslie A. Emmert for his suggestion of the problem, his patience, encouragement and supervision throughout the course of this thesis. His guidance helped overcome many problems which arose during the course of this study. I also wish to express thanks to Dr. Charles C.C. O'Morchoe and Dr. Maurice V. L'Heureux for their many valuable suggestions during the writing of this thesis. -

Preparation and Ultra-Violet Absorption Studies of Leucocyanides of the Triarylmethane Dyes
University of the Pacific Scholarly Commons University of the Pacific Theses and Dissertations Graduate School 1960 Preparation and ultra-violet absorption studies of leucocyanides of the triarylmethane dyes Arthur Katzakian Jr. University of the Pacific Follow this and additional works at: https://scholarlycommons.pacific.edu/uop_etds Part of the Chemistry Commons Recommended Citation Katzakian, Arthur Jr.. (1960). Preparation and ultra-violet absorption studies of leucocyanides of the triarylmethane dyes. University of the Pacific, Thesis. https://scholarlycommons.pacific.edu/uop_etds/ 1471 This Thesis is brought to you for free and open access by the Graduate School at Scholarly Commons. It has been accepted for inclusion in University of the Pacific Theses and Dissertations by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. 1..J H.t:£1ArtATluN AN D Ul.il h A-VluLB~' AJ3 5v!·. r'1'10.14 SliUDI .:; ..) A Theeis Submi ·ttea to the .Fo. oul r.y of the College of the h.toific f or the Degre t1 of .Mt.. ater of Soieuoe in the Depo.rtment of Ohemi stry by Arthur Ku... tzukil-ln , J1· • ACK NOW.L EDGE ME NT The writer wishes to express his appr e cia tion to the membe1·s of the f C~.c ul ty of t he Chemistry De pe:.rtment of the College of the Pacific f or t heix- e.o sistance during the period of study and es pecially t o expresb his gr a titude to Dr·. Hugh Wl:idman and Dr . Emerson Cobb f or their guidance aHd criticisms in t his wor k . -

Reticulin Stain
US 20110229879A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0229879 A1 CHURUKIAN (43) Pub. Date: Sep. 22, 2011 (54) METHODS AND COMPOSITIONS FOR Publication Classification NUCLEAR STANING (51) Int. Cl. (75) Inventor: Charles J. CHURUKIAN, CI2O I/68 (2006.01) Rochester, NY (US) (52) U.S. Cl. ......................................................... 435/6.1 (73) Assignee: UNIVERSITY OF ROCHESTER, Rochester, NY (US) (57) ABSTRACT The present invention relates to compositions, methods, and (21) Appl. No.: 13/052,791 kits Suitable for detecting nucleic acids inabiological sample. The nuclear staining composition of the present invention (22) Filed: Mar. 21, 2011 contains a pH buffering reagent, a solubilizing reagent, a basic dye, and an aqueous medium. The composition can be Related U.S. Application Data used alone to detect nucleic acids in a biological sample or in (60) Provisional application No. 61/315,483, filed on Mar. combination with other histological dyes for nuclear counter 19, 2010. staining. Reticulin Stain Patent Application Publication Sep. 22, 2011 Sheet 1 of 3 US 2011/0229879 A1 Reticulin Stain Figures 1A-1B Patent Application Publication Sep. 22, 2011 Sheet 2 of 3 US 2011/0229879 A1 Iron Stain Figures 2A-2B Patent Application Publication Sep. 22, 2011 Sheet 3 of 3 US 2011/0229879 A1 Alcian Blue Figures 3A-3B US 2011/0229.879 A1 Sep. 22, 2011 METHODS AND COMPOSITIONS FOR composition of the present invention does not precipitate in NUCLEAR STANING Solution and has a shelf-life of at least one year. In addition, nuclear staining with the composition of the present invention achieves brighter, more brilliant nuclear staining showing 0001. -

Tissue in Cases of Acute Rheumatism
Ann Rheum Dis: first published as 10.1136/ard.16.3.307 on 1 September 1957. Downloaded from Ann. rheum. Dis. (1957), 16, 307. HISTOCHEMICAL INVESTIGATION OF CONNECTIVE TISSUE IN CASES OF ACUTE RHEUMATISM BY A. I. STRUKOV AND G. V. ORLOVSKAYA U.S.S.R. Academy of Medical Sciences, Moscow (RECEIVED FOR PUBLICATION JULY 5, 1957) The connective tissue is the chief place where the phases: soluble (procollagen) and insoluble (colla- pathological rheumatic process takes place. The stromine). Obtained by precipitation from a changes mainly affect its intercellular components. solution, procollagen has all the properties of On the basis of this localization of the pathological collagen fibrils (of collagen): it turns red when it is lesions, rheumatism has been included among the stained by Van Gieson stain, and its roentgenogram so-called collagen diseases (Klemperer, 1950). The shows the characteristic interplane intervals of mechanism of development of the pathological 2-9 A and 11-0 A. Under the electron microscope by copyright. processes in the intercellular substance of connective it shows the same transverse bands. tissue can be understood only when there is a clear In a roentgenogram, collastromine shows no idea of the nature, structure, and correlations of ring at 2 9 A which is characteristic for collagen; collagen and the amorphous cementing substance, it has no periodicity, does not turn red with Van both of which play a principal role in the develop- Gieson stain, and shows a distinct metachromasia; ment of the rheumatic process. staining with silver reveals argyrophilic fibres. The The investigations made in recent years with the results of fractioning show collagen as at least a aid of physical, chemical, and histochemical bi-phasic system; the known features of collagen methods have considerably advanced our concepts in toto are determined by its content of procollagen. -

Magenta and Magenta Production
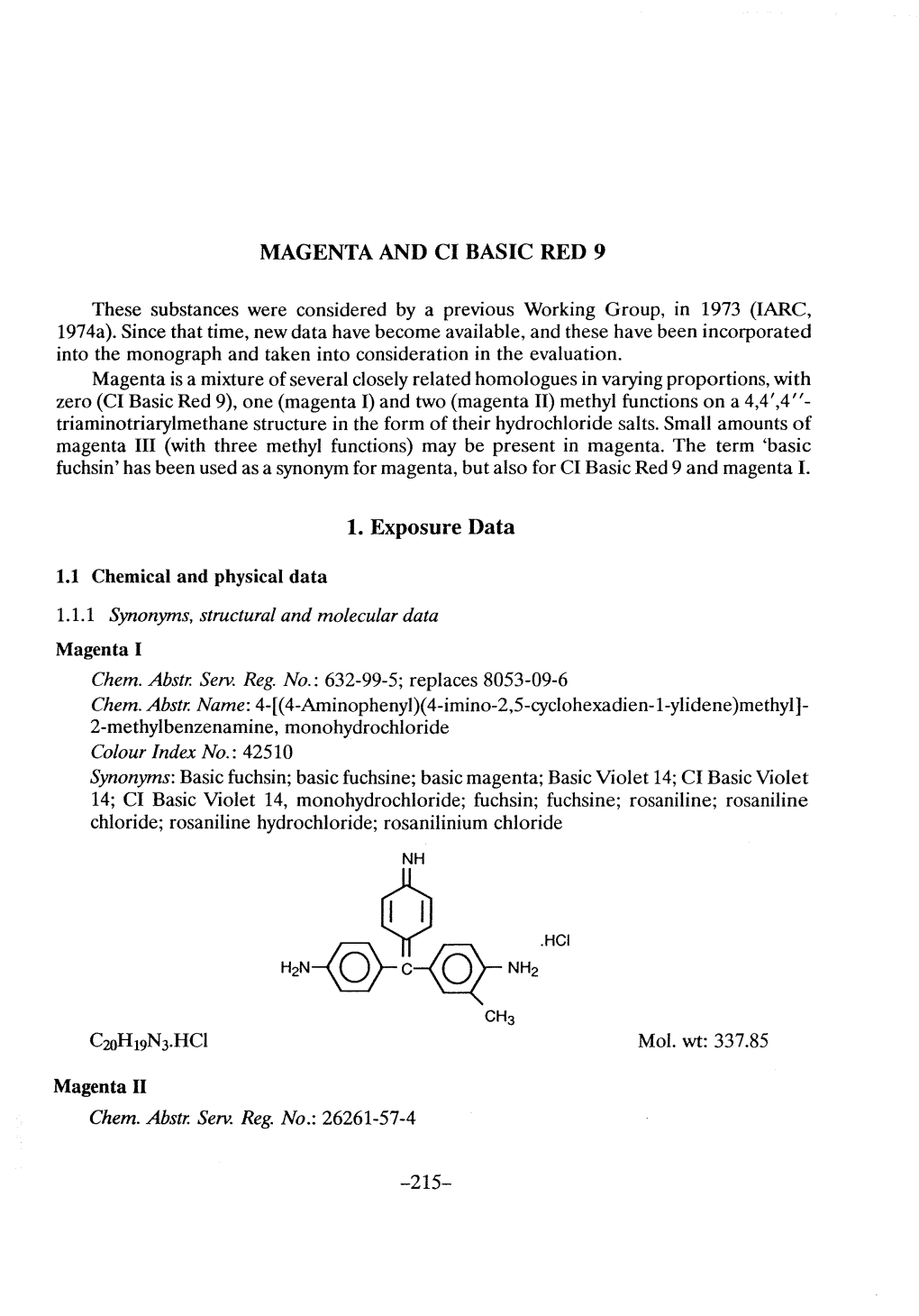
MAGENTA AND MAGENTA PRODUCTION Historically, the name Magenta has been used to refer to the mixture of the four major constituents comprising Basic Fuchsin, namely Basic Red 9 (Magenta 0), Magenta I (Rosaniline), Magenta II, and Magenta III (New fuchsin). Although samples of Basic Fuchsin can vary considerably in the proportions of these four constituents, today each of these compounds except Magenta II is available commercially under its own name. Magenta I and Basic Red 9 are the most widely available. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Magenta I (a) Nomenclature Chem. Abstr. Serv. Reg. No.: 632–99–5 CAS Name: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, hydrochloride (1:1) Synonyms: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, monohydrochloride; Basic Fuchsin hydrochloride; C.I. 42510; C.I. Basic Red; C.I. Basic Violet 14; C.I. Basic Violet 14, monohydrochloride; 2- methyl-4,4'-[(4-imino-2,5-cyclohexadien-1-ylidene)methylene]dianiline hydrochloride; rosaniline chloride; rosaniline hydrochloride –297– 298 IARC MONOGRAPHS VOLUME 99 (b) Structural formula, molecular formula, and relative molecular mass NH HCl H2N NH2 CH3 C20H19N3.HCl Rel. mol. mass: 337.85 (c) Chemical and physical properties of the pure substance Description: Metallic green, lustrous crystals (O’Neil, 2006; Lide, 2008) Melting-point: Decomposes above 200 °C (O’Neil, 2006; Lide, 2008) Solubility: Slightly soluble in water (4 mg/mL); soluble in ethanol (30 mg/mL) and ethylene -

United States Patent Office Patented Jan
3,488,705 United States Patent Office Patented Jan. 6, 1970 2 3,488,705 having desirable electrophotographic properties can be THERMALLY UNSTABLE ORGANIC ACD SALTS especially useful in elecetrophotography. Such electro OF TRARYLMETHANE DYES AS SENSTEZERS photographic elements may be exposed through a trans FOR ORGANIC PHOTOCONDUCTORS parent base if desired, thereby providing unusual flexibility Charles J. Fox and Arthur L. Johnson, Rochester, N.Y., in equipment design. Such compositions, when coated as assignors to Eastman Kodak Company, Rochester, a film or layer on a suitable support also yield an element N.Y., a corporation of New Jersey No Drawing. Continuation-in-part of application Ser. No. which is reusable; that is, it can be used to form subse 447,937, Mar. 16, 1965. This application Dec. 4, 1967, quent images after residual toner from prior images has Ser. No. 687,503 been removed by transfer and/or cleaning. nt. C. G03g 5/06, 9/00 0. Although some of the organic photoconductors com U.S. C. 96-1.6 32 Claims prising the materials described are inherently light sensi tive, their degree of sensitivity is usually low and in the short wavelength portion of the spectrum so that it is ABSTRACT OF THE DISCLOSURE common practice to add materials to increase the speed 15 and to shift the sensitivity toward the longer wavelength Organic acid salts of triarylmethane dyes are useful as portion of the visible spectrum. Increasing the speed and sensitizers in electrophotographic elements. They are shifting the sensitivity of such systems into the visible thermally unstable and thus readily bleachable.