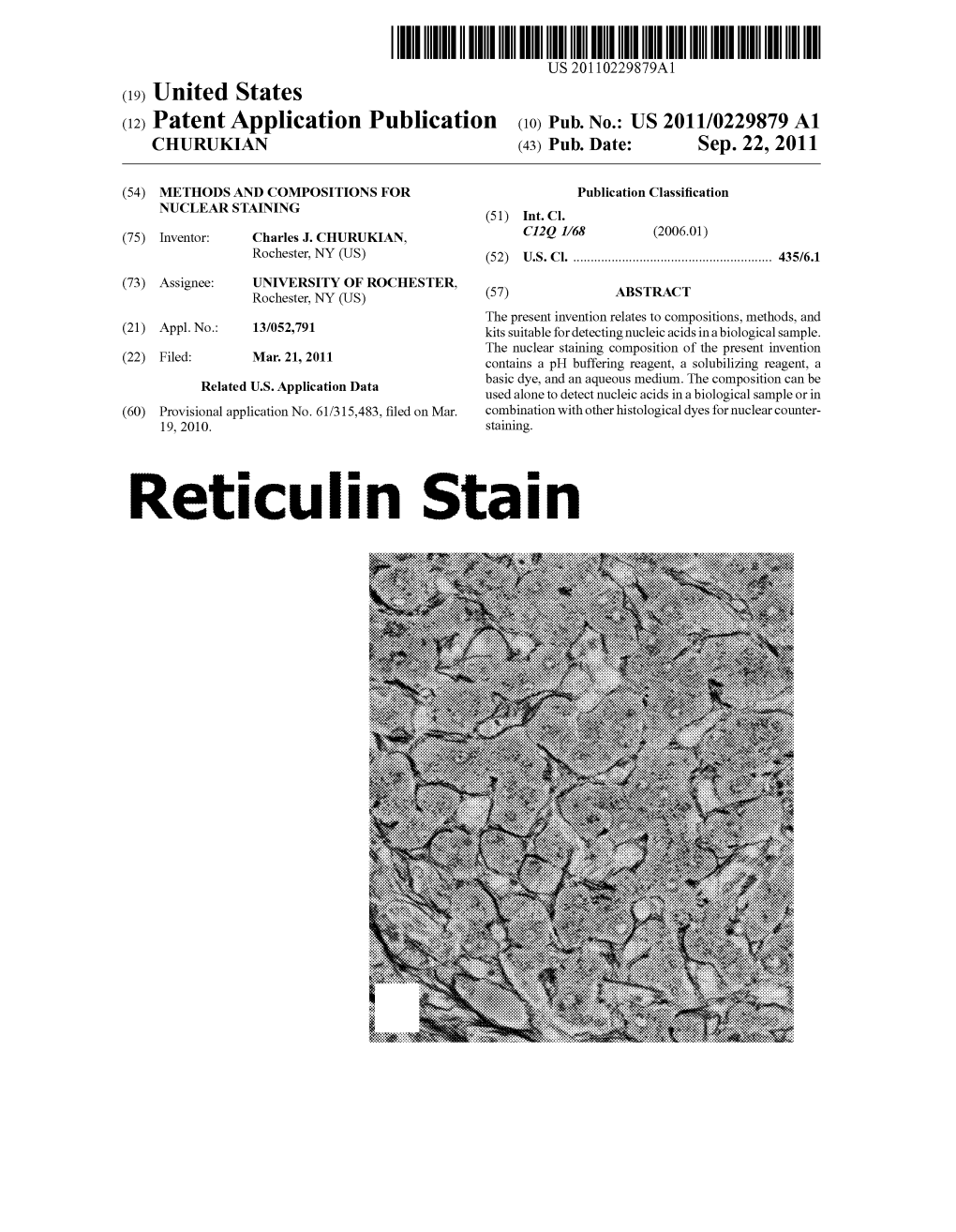
Reticulin Stain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21
Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21 भारत सरकार Government of India स्वास्थ्य सेवा महानिदेशालय Directorate General of Health Services स्वास्थ्य एवं पररवार कल्याण मंत्रालय Ministry of Health & Family Welfare ग मेनडकल कॉलेज एवं श्रीमती सुचेता कृपलािी अस्पतालﴂलेडी हनड Lady Hardinge Medical College & Smt. Sucheta Kriplani Hospital शहीद भगत नसंह मागग, िई नदल्ली – ११०००१ Shaheed Bhagat Singh Marg, New Delhi-110001 ३ नसतम्बर २०२० / 3rd September 2020 भंडार अिुभाग/Stores Section Tender Documents for Advertised Tender Enquiry for running rate contract of Kits, Chemicals & Reagents required for Lady Hardinge Medical College & Associated Hospitals New Delhi (Two Bid System) Tender Enquiry No: 8-61/Stores/LHMC/AT/2020-21 Dated: 3rd September 2020 Amount of Bid Security: Rs. 2,00,000.00 (Rs. Two Lakh Only) Tender Fee: Rs. 0 (Can be downloaded from Central Public Procurement Portal or LHMC Website) CRITICAL DATES Start Date of Sale of Tender: 04/09/2020 11.00 AM to 1.30 PM and from 2.30 PM to 4.00 PM End Date of Sale of Tender: 12/10/2020 4.00 PM Start Date for Submission of Tender: 13/10/2020, 10.00 AM onwards, Time Schedule for Submission of Tender: 14/10/2020, upto 11.00 AM Time Schedule for Opening of Tender: 14/10/2020, 11.30 AM A. INSTRUCTIONS 1. LHMC & Associated Hospitals proposed to enter into a rate-contract (R/C) for the supply of Chemicals, Reagents & Kits valid for a period of 24 months from the date of opening of the Price Bid, can be extended for a period of 12 months or more on mutual consent on existing terms & conditions. -

Magenta and Magenta Production
MAGENTA AND MAGENTA PRODUCTION Historically, the name Magenta has been used to refer to the mixture of the four major constituents comprising Basic Fuchsin, namely Basic Red 9 (Magenta 0), Magenta I (Rosaniline), Magenta II, and Magenta III (New fuchsin). Although samples of Basic Fuchsin can vary considerably in the proportions of these four constituents, today each of these compounds except Magenta II is available commercially under its own name. Magenta I and Basic Red 9 are the most widely available. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Magenta I (a) Nomenclature Chem. Abstr. Serv. Reg. No.: 632–99–5 CAS Name: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, hydrochloride (1:1) Synonyms: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, monohydrochloride; Basic Fuchsin hydrochloride; C.I. 42510; C.I. Basic Red; C.I. Basic Violet 14; C.I. Basic Violet 14, monohydrochloride; 2- methyl-4,4'-[(4-imino-2,5-cyclohexadien-1-ylidene)methylene]dianiline hydrochloride; rosaniline chloride; rosaniline hydrochloride –297– 298 IARC MONOGRAPHS VOLUME 99 (b) Structural formula, molecular formula, and relative molecular mass NH HCl H2N NH2 CH3 C20H19N3.HCl Rel. mol. mass: 337.85 (c) Chemical and physical properties of the pure substance Description: Metallic green, lustrous crystals (O’Neil, 2006; Lide, 2008) Melting-point: Decomposes above 200 °C (O’Neil, 2006; Lide, 2008) Solubility: Slightly soluble in water (4 mg/mL); soluble in ethanol (30 mg/mL) and ethylene -

United States Patent Office Patented Jan
3,488,705 United States Patent Office Patented Jan. 6, 1970 2 3,488,705 having desirable electrophotographic properties can be THERMALLY UNSTABLE ORGANIC ACD SALTS especially useful in elecetrophotography. Such electro OF TRARYLMETHANE DYES AS SENSTEZERS photographic elements may be exposed through a trans FOR ORGANIC PHOTOCONDUCTORS parent base if desired, thereby providing unusual flexibility Charles J. Fox and Arthur L. Johnson, Rochester, N.Y., in equipment design. Such compositions, when coated as assignors to Eastman Kodak Company, Rochester, a film or layer on a suitable support also yield an element N.Y., a corporation of New Jersey No Drawing. Continuation-in-part of application Ser. No. which is reusable; that is, it can be used to form subse 447,937, Mar. 16, 1965. This application Dec. 4, 1967, quent images after residual toner from prior images has Ser. No. 687,503 been removed by transfer and/or cleaning. nt. C. G03g 5/06, 9/00 0. Although some of the organic photoconductors com U.S. C. 96-1.6 32 Claims prising the materials described are inherently light sensi tive, their degree of sensitivity is usually low and in the short wavelength portion of the spectrum so that it is ABSTRACT OF THE DISCLOSURE common practice to add materials to increase the speed 15 and to shift the sensitivity toward the longer wavelength Organic acid salts of triarylmethane dyes are useful as portion of the visible spectrum. Increasing the speed and sensitizers in electrophotographic elements. They are shifting the sensitivity of such systems into the visible thermally unstable and thus readily bleachable. -

The Color of Tissue Diagnostics Routine Stains, Special Stains and Ancillary Reagents
The Color of Tissue Diagnostics Routine Stains, Special Stains and Ancillary Reagents The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. For over years, 100routine stains, special stains and ancillary reagents have been part of the MilliporeSigma product range. This tradition and experience has made MilliporeSigma one of the world’s leading suppliers of microscopy products. The products for microscopy, a comprehensive range for classical hematology, histology, cytology, and microbiology, are constantly being expanded and adapted to the needs of the user and to comply with all relevant global regulations. Many of MilliporeSigma’s microscopy products are classified as in vitro diagnostic (IVD) medical devices. Quality Means Trust As a result of MilliporeSigma’s focus on quality control, microscopy products are renowned for excellent reproducibility of results. MilliporeSigma products are manufactured in accordance with a quality management system using raw materials and solvents that meet the most stringent quality criteria. Prior to releasing the products for particular applications, relevant chemical and physical parameters are checked along with product functionality. The methods used for testing comply with international standards. For over Contents Ancillary Reagents Microbiology 3-4 Fixing Media 28-29 Staining Solutions and Kits years, 5-6 Embedding Media 30 Staining of Mycobacteria 100 6 Decalcifiers and Tissue Softeners 30 Control Slides 7 Mounting Media Cytology 8 Immersion -
Magenta and CI Basic Red 9 Are of Briliant Hue, Exhibit High Tinctorial Strength, Are Relatively Inexpensive and May Be Applied to a Wide Range of Substrates
MAGENTA AND ei BAsie RED 9 These substances were considered by a previous Working Group, in 1973 (lARe, 1974a). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the evaluation. Magenta is a mixture of several closely related homologues in varyng proportions, with zero (CI Basic Red 9), one (magenta 1) and two (magenta II) methyl functions on a 4,4',4"- triaminotriarylmethane structure in the form of their hydrochloride salts. Small amounts of magenta III (with three methyl functions) may be present in magenta. The term 'basic fuchsin' has been used as a synonym for magenta, but also for Ci Basic Red 9 and magenta I. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Magenta 1 Chem. Abstr. Sem Reg. No.: 632-99-5; replaces 8053-09-6 Chem. Abstr. Name: 4-(( 4-Aminophenyl)( 4-imino-2,5-cyclohexadien- 1 -ylidene )methyl)- 2-methylbenzenamine, monohydrochloride Colour Index No.: 42510 Synonyms: Basic fuchsin; basic fuchsine; basic magenta; Basic Violet 14; CI Basic Violet 14; Ci Basic Violet 14, monohydrochloride; fuchsin; fuchsine; rosaniline; rosaniline chloride; rosaniline hydrochloride; rosanilinium chloride NH H~-\C~Q .HCINH2 CH3 C20H19N3.HCI MoL. wt: 337.85 Magenta II Chem. Abstr. Sem Reg. No.: 26261-57-4 -215- 216 IAC MONOGRAHS VOLUME 57 Chem. Abstr. Name: 4-( (4-Aminophenyl)( 4-imino-3-methyl-2,5-cyclohexadien- 1- ylidene )methyll-2-methylbenzenamine, monohydrochloride Synonym: Dimethyl fuchsin NH ~CH3 H2N~ y/e-\ .HeiNH2 CH3 C2iH2iN3.HCI MoL. wt: 351.9 Magenta III Chem. -

REPORTS of the Scientific Committee on Cosmetology (Seventh Series)
Commission of the European Communities environment and quality of life REPORTS of the Scientific Committee on Cosmetology (seventh series) Commission of the European Communities environment and quality of life REPORTS of the Scientific Committee on Cosmetology (seventh series) r PARL. EUROP. BHioth. Directorate-General Environment, Consumer Protection and Nuclear í jfefe/C0M*frfr3S- 7 1988 CL EUR 11303 EN I Published by the COMMISSION OF THE EUROPEAN COMMUNITIES Directorate-General Telecommunications, Information Industries and Innovation Bâtiment Jean Monnet LUXEMBOURG LEGAL NOTICE Neither the Commission of the European Communities nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information Cataloguing data can be found at the end of this publication Luxembourg: Office for Official Publications of the European Communities, 1988 ISBN 92-825-8129-2 Catalogue number: CD-NA-11303-EN-C © ECSC-EEC-EAEC, Brussels • Luxembourg, 1988 Printed in Belgium FOREWORD The Scientific Committee on Cosmetology was set up by Commission Decision 78/45/EEC on 19 December 1977 (OJ N° L 13 of 17 January 1978, p. 24) in order to provide the Commission with informed opinions on any scientific and technical problems arising in connection with cosmetic products, and in particular on the substances used in their manufacture, on their composition and on the conditions for their use. The members of the Committee are independent scientists highly qualified in the fields of medicine, toxicology, biology, chemistry or other similar di sci pli nes. The Committee is serviced by the Directorate-general for the environment, consumer protection and nuclear safety. -

Benchmark Special Stains Product Guide
BenchMark Special Stains Product guide 6085A_Product_guide_BMK_SS_field_guide_V3_Germany.indd 1 6/27/2016 2:18:40 PM Quick reference table Ordering Catalog Tissue Tests Total vials in kit Vials run on Minimum runtime Maximum runtime Product name code number thickness per kit package instrument (minutes) including depar** (minutes) including depar** AFB III Staining Kit 05279437001 860-027 3-5 µm 75 tests 3 3 52 60 Alcian Blue Staining Kit 05279186001 860-002 3-5 µm 75 tests 2 2 44 72 Alcian Yellow Staining Kit 05279321001 860-017 3-5 µm 75 tests 7* 5 68 68 Congo Red Staining Kit 05279429001 860-026 6-10µm 40 tests 3 3 65 85 Diastase Kit 05279208001 860-004 3-5 µm 75 tests 1 1+PAS staining kit 83 110 Elastic Staining Kit 05279216001 860-005 3-5 µm 75 tests 5 5 67 75 Giemsa Staining Kit 05279224001 860-006 3-5 µm 75 tests 1 1 41 41 GMS II Staining Kit 05412749001 860-028 3-5 µm 75 tests 8* 7 124 136 Gram Staining Kit 06890105001 860-039 3-5 µm 75 tests 6 6 80 92 Iron Staining Kit 05279259001 860-009 3-5 µm 75 tests 3 3 48 60 Jones H&E Staining Kit 05279348001 860-019 2-4µm 40 tests 7 7 100 128 Jones Light Green Staining Kit 05279356001 860-020 2-4µm 40 tests 7 7 100 132 Mucicarmine Staining Kit 05279275001 860-011 3-5 µm 75 tests 4 4 56 84 PAS - Alcian Blue 05279194001 860-003 3-5 µm 75 tests 1 1+PAS staining kit 68 84 PAS - Light Green 05279267001 860-010 3-5 µm 75 tests 1 1+PAS staining kit 68 80 PAS Staining Kit 05279291001 860-014 3-5 µm 75 tests 5* 3 69 85 Reticulum II Staining Kit 05279399001 860-024 3-5 µm 75 tests 8 8 112 120 Steiner II Staining Kit 06521894001 860-030 3-5 µm 40 tests 9 8 116 124 Green for Trichrome 06521916001 860-032 3-5 µm 75 tests 1 1+Trichrome staining kit 140 228 Trichrome Staining Kit 06521908001 860-031 3-5 µm 60 tests 7 7 140 228 *Not all vials in the kit are used at one time, e.g., PAS includes three vials of Schiff’s reagent.