Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study of Rawitz's 'Inversion Staining' by ALEKSANDRA PRZEL^CKA
231 A Study of Rawitz's 'Inversion Staining' By ALEKSANDRA PRZEL^CKA {From the Cytological Laboratory, Department of Zoology, University Museum, Oxford, and the Nencki Institute, 3 Pasteur St., Warsaw 22; present address, Nencki Institute) SUMMAHY The Rawitz method involves mordanting with tannic acid and potassium antimony tartrate, and staining with basic fuchsine. The mordanting causes basic fuchsine to act as though it were an acid dye ('inversion staining'). A modification of the method is described in the present paper. This modification makes it possible to obtain the same results in a shorter time. The chief substances stained by Rawitz's method are phospholipids, certain pro- teins, and certain polysaccharides. Although the method cannot be regarded as a cytochemical test in the strict sense, yet it gives useful indications of chemical composition and in addition is valuable to the morphological cytologist as a technique for showing certain cytoplasmic inclusions (mitotic spindle, acrosome, mitochondria, 'Golgi apparatus' of certain cells). INTRODUCTION T is well known that the so-called 'Golgi apparatus' is extremely difficult to I reveal by any staining method. Baker, in the course of his investigation on this organelle in the epididymis of the mouse, found that it can be stained by basic fuchsin after a special mordanting process (1957). The method was taken from Rawitz (1895), who found that basic fuchsin, if mordanted with tannic acid and potassium antimony tartrate, loses the character of a dye for chro- matin and colours the cytoplasm instead. Rawitz called this effect 'inversion staining'. Since this technique, when applied to various kinds of cytological material, gave good selectivity in visualizing certain delicate cell structures, it seemed interesting to investigate the nature of the chemical compounds which are responsible for positive Rawitz staining. -

Revisions Inserts Rev from Rev to JOB
BALTSO0191 Version 11.0 Template 4 Revisions Inserts Rev from Rev to JOB # 06 07 52-17 Notes: 1. BD Catalog Number: 212525, 212526, 212527, 212528, 212531, 212532, 212539, 212542, 212543, 212544, 212545 2. Blank (Sheet) Size: Length: 25.5” Width: 22” 3. Number of Pages: 28 Number of Sheets: 1 4. Page Size: Length: 8.5” Width: 5.5” Final Folded Size: 4.25” x 5.5” 5. Ink Colors: No. of Colors: 2 PMS#: 032 Red; Standard Black 6. Printed two sides: Yes X No 7. Style (see illustrations below): # 5 W W W W W W W 8. Vendor Printed X Online/In House Printed Web 9. See specication control no. N/A for material information. 10. Graphics are approved by Becton, Dickinson and Company. Supplier has the responsibility for using the most current approved revision level. Label Design COMPANY CONFIDENTIAL. THIS DOCUMENT IS THE PROPERTY OF BECTON, DICKINSON AND Becton, Dickinson and Company Proofer COMPANY AND IS NOT TO BE USED OUTSIDE THE COMPANY WITHOUT WRITTEN PERMISSION. 7 Loveton Circle Sparks, MD 21152 USA Checked By Category and Description Sheet: 1 of 29 Part Number: Package Insert, 8820191JAA Gram Stain Kits and Reagents Scale: N/A A B Gram Stain Kits and Reagents English: pages 1 – 5 Italiano: pagine 14 – 18 8820191JAA(07) Français : pages 5 – 9 Español: páginas 19 – 23 2017-09 Deutsch: Seiten 10 – 14 Contact your local BD representative for instructions. / Свържете се с местния представител на BD за инструкзии. / Pokyny vám poskytne místní zástupce společnosti BD. / Kontakt den lokale BD repræsentant for at få instruktioner. -

Factors Affecting the Adsorption of Some Ionic Dyes on the Surface of Modify Cao from Eggshell
Asian Journal of Applied Sciences (ISSN: 2321 – 0893) Volume 07 – Issue 01, February 2019 Factors Affecting the Adsorption of Some Ionic Dyes on the Surface of Modify CaO from Eggshell Ibtighaa K. Radhi, Mouayed A. Hussein, Zaki N. Kadhim* Department of Chemistry, College of Science, University of Basrah Basrah, Iraq *Corresponding author’s emails: zekinasser99 [AT] yahoo.com ________________________________________________________________________________________________ ABSTRACT--- In this paper, calcium oxide (CaO) was produced by the thermal treatment of eggshell. The doping process with silver iodide (AgI), oxygen (O), sulfur(S) and nitrogen (N) was achieved by adsorbents. The adsorption of Acid fuchsine (AF), Indigo Carmine (IC), Nigrosine (NG) and Alizarine Red S (AR) on the surface of these particles was studied. The different conditions affecting the adsorption process, such as the time of equilibrium, the primary concentration of the studied dyes, the amount of the adsorbent, the acidic function, the speed of the pruning motion and the temperature were studied. The pH stability time (5-10 minutes), IC and NG (30 minutes) and AR were (90 minutes). The effect of temperature was also studied within the range (25-45 ° C). The results showed that the adsorption capacity increased by increasing the temperature, ie the reaction is endothermic. The study showed the effect of the acidic function on the percentage of pigmentation. The percentage was increased by increasing the acidic function in the basal circles on the surfaces except for the AR dye. It decreased the percentage by increasing the acidic function. The effect of the weight of the adsorbent was studied on the percentage of adsorption. -

Magenta and Magenta Production
MAGENTA AND MAGENTA PRODUCTION Historically, the name Magenta has been used to refer to the mixture of the four major constituents comprising Basic Fuchsin, namely Basic Red 9 (Magenta 0), Magenta I (Rosaniline), Magenta II, and Magenta III (New fuchsin). Although samples of Basic Fuchsin can vary considerably in the proportions of these four constituents, today each of these compounds except Magenta II is available commercially under its own name. Magenta I and Basic Red 9 are the most widely available. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Magenta I (a) Nomenclature Chem. Abstr. Serv. Reg. No.: 632–99–5 CAS Name: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, hydrochloride (1:1) Synonyms: 4-[(4-Aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2- methylbenzenamine, monohydrochloride; Basic Fuchsin hydrochloride; C.I. 42510; C.I. Basic Red; C.I. Basic Violet 14; C.I. Basic Violet 14, monohydrochloride; 2- methyl-4,4'-[(4-imino-2,5-cyclohexadien-1-ylidene)methylene]dianiline hydrochloride; rosaniline chloride; rosaniline hydrochloride –297– 298 IARC MONOGRAPHS VOLUME 99 (b) Structural formula, molecular formula, and relative molecular mass NH HCl H2N NH2 CH3 C20H19N3.HCl Rel. mol. mass: 337.85 (c) Chemical and physical properties of the pure substance Description: Metallic green, lustrous crystals (O’Neil, 2006; Lide, 2008) Melting-point: Decomposes above 200 °C (O’Neil, 2006; Lide, 2008) Solubility: Slightly soluble in water (4 mg/mL); soluble in ethanol (30 mg/mL) and ethylene -

Marking Tephritidae Fruit Fly Adults in Hawaii for Release-Recovery
Vol. XXIII, No. 3, February, 1981 437 Marking Tephritidae Fruit Fly Adults in Hawaii for Release-Recovery Studies1234 W. J. SCHROEDER5AND W. C. MlTCHELL6 The need to identify released insects is paramount in studies based on release-recovery evaluations. In Hawaii identification of released oriental fruit flies, Dacus dorsalisHendel, is accomplished by rearing and releasing phenotypically distinct flies (Steiner et al, 1962). Presently, marking the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), and the melon fly, D. cucurbitae Coquillett, is accomplished with the use of Calco Blue ® dye (Steiner, 1965), Blaze Orange Day-Glo® fluorescent dye (Holbrook et al, 1970) or Tinopal® SFG fluorescent whitening agent (Schroeder et aL, 1972). Because of the need for additional markers, we routinely examine dye materials as possible external (ptilinum dye) and internal (vital dye) markers. These continuing studies include coating pupae with dye powder to determine the potential of the material as an external adult dye and addition of the dye to the larval diet followed by examination of the adult to determine the potential of the material as a vital dye. Promising mate rials are then evaluated for retention by the insect and ease of detection, and dyed insects are examined for behavioral changes. Finally the material is evaluated in a field release-recovery test. This report describes the general procedure used in dyeing and examining adult flies for the marker, lists materials tested, and discusses dyes presently used in Hawaii. General.—Adult Tephritidae are marked with an external dye by tum bling a known quantity of pupae with a known quantity of dry powder. -

Histology Stain Manual Open Educational Resource
DE MONTFORT UNIVERSITY School of Allied Health Sciences Histology Stain Manual Open Educational Resource http://www.val.biologycourses.co.uk Handbook updated last August 2009 Many of these recipes are freely available on the internet these days, and the ones in this book were used in student classes and have been tried and tested over the years. 1. The aim of this handbook……………………. 2. Introduction to this handbook…. 3. Major changes to previous versions….. 4. ALCIAN BLUE-PAS COMBINED TECHNIQUE….. 5. ALCIAN BLUE-CRITICAL ELECTROLYTE CONCENTRATIONS….. 6. ALDEHYDE-FUCHSINE 7 FEULGEN-SCHIFF'S REACTION for DEOXYRIBONUCLEIC ACID (DNA) 8. GIEMSA STAIN (for cytological/haematological smears)….. 9. GIEMSA STAIN (post-dichromate fixation)….. 10. GORDON AND SWEET’S RETICULIN METHOD….. 11. HAEMATOXYLIN AND EOSIN….. 12. LUXOL FAST BLUE (Normal Myelin/ Lipofuscins))…. 13. MARTIUS, SCARLET, BLUE (M.S.B.)…. 14. MASSON'S TRICHROME TECHNIQUE…. 15. MAYER’S HAEMATOXYLIN AND EOSIN…. 16. PALMGREN’S METHOD (modified) for NERVE FIBRES…. 17. PERIODIC ACID-SCHIFF (PAS) REACTION…. 18. SCOTT’S TAP WATER…. 19. SOUTHGATE’S MUCICARMINE METHOD…. 20. THE ROMANOWSKY STAINS (Romanowsky 1891)…. 21. TOLUIDINE BLUE… 22. VAN GIESON’S STAIN FOR COLLAGEN…. 23. Resources…. 2 1. The aim of this handbook……………………. This stain manual is an essential laboratory guide for biomedical science and similar degree programmes where students need to master a number of histochemical stains. All the stains have been used in student classes so are tried and tested, although as you probably appreciate, a number of alternate recipes are available and will be equally as good. 2. Introduction to this handbook…. Histological staining techniques are fundamental to any research laboratory, whether it is in a hospital, pharmaceutical company or other industrial setting. -

Handbook of Biological Dyes and Stains Synthesis and Industrial Applications
HANDBOOK OF BIOLOGICAL DYES AND STAINS SYNTHESIS AND INDUSTRIAL APPLICATIONS R. W. SABNIS Pfizer Inc. Madison, NJ HANDBOOK OF BIOLOGICAL DYES AND STAINS HANDBOOK OF BIOLOGICAL DYES AND STAINS SYNTHESIS AND INDUSTRIAL APPLICATIONS R. W. SABNIS Pfizer Inc. Madison, NJ Copyright Ó 2010 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, exckpt as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization though payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 kver Street, Hoboken, NJ 07030, (201) 748-601 1, fax (201) 748-6008, or online at http://www.wiley.com/go/permission. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. -
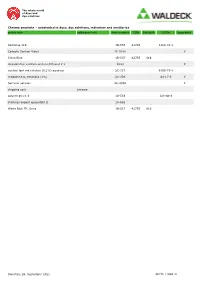
Article Text Additional Text Cinr Schulznr Casnr Item Number
The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous Acid blue 119 1B-555 42765 1324-76-1 Carbolic Gentian Violet 2E-028K Y China Blue 1B-507 42755 816 discoloration solution acetone/Ethanol 1:1 E333 Y nuclear fast red solution (0,1%) aqueous 2C-337 6409-77-4 rhodamine b, ethanolic (1%) 2C-339 64-17-5 Y Safranin solution 2C-333K Y shipping cost chroma solvent green 3 1B-553 128-80-3 staining reagent eppendahl II 1A-652 Water Blue TR, Unna 1B-517 42755 816 Dienstag, 28. September 2021 SEITE 1 VON 21 The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous dyes Acid Alizarine Blue B 1A-252 16680 1058-92-0 Acid Black 12 B 1A-598 20470 299 1064-48-8 acid brilliant flavine 7g 1F-562 61968-07-8 Acid Fuchsine-Orange 1F-347 Acid Green G 1B-215 42095 765 5141-20-8 Acid Rhodamine 1A-004 45100 863 3520-42-1 Acridine Orange 3 R zinc chloride double salt 1B-307 46005 10127-02-3 Acridine Yellow 1B-331 46025 135-49-9 Acriflavine 5A-406 46000 906 Y Alcian Green 2 GX 1F-555 Alcian Green 3 BX 1F-551 Alcian Yellow GXS 1F-597 12840 61968-76-1 Alizarine Blue B 1A-246 16680 Alizarine Brilliant Violet R 1B-077 60730 1196 4430-18-6 Alizarine Carmine 1F-581 58005 1145 130-22-3 Alizarine Pure 1A-020 58000 1141 72-48-0 Alizarine Purple RS 1B-079 60730 1196 4430-18-6 Alizarine Red S 1F-583 58005 -

1422-ABS-Methyl Violet-Fuchsine-Mdk
The oldest synthetic organic dyestuff group: the history and analysis of the triarylmethane dyes Matthijs de Keijzer, Maarten van Bommel Netherlands Institute for Cultural Heritage, Department: Research P.O. Box 76709, NL-1070 KA, Amsterdam, The Netherlands [email protected] , [email protected] Regina Hofmann-de Keijzer University of Applied Arts Vienna, Department Archaeometry Salzgries 14/1, A-1013 Vienna, Austria [email protected] Abstract The present lecture focuses on the so-called triarylmethanes. This dyestuff class is the oldest synthetic organic group and produces brilliant hues, the range including reds, violets, blues and greens. During the second half of the 19 th century the triarylmethane dyes played an important role for dyeing textiles and for other applications. The history of this dyestuff group is studied by the original historical sources, included the patent literature. The start of this group was the discovery of the red-violet dye fuchsine, which was developed in the same year of the discovery of mauve but turned out to be much more important. In 1856 the Polish chemist Jakub Natanson (1832-1884) and two years later August Wilhelm Hofmann (1818-1892) obtained a red-violet dyestuff by using aniline. They were not aware of the use as a textile dye. In the same year François-Emmanuel Verguin (1814-1864), professor on the Collège of Lyon, developed a method to produce this dye, but he did not exploit this invention by himself. The production process came into the hand of the silk dyers Renard Frères & Franc in Lyon. -

On the Function of the Casparian Strips in Roots
Acta Bot. Neerl. 23(2), April 1974, p. 171-175. On the function of the Casparian strips in roots B. Huisinga and A.M.W. Knijff Botanisch Laboratorium, Utrecht SUMMARY The dyes safranin and basic fuchsine as well as ferric salts enter the stele when applied as dilute solutions to intactroot parts where Casparian strips are well developed.As the entrance is not hamperedwhen the protoplasm has been killed or its activity and permeability dimin- ished by low temperaturethe most likely way ofentrance is throughthe cell walls. In the Casparian strips no fatty substances could be detected. As soon as traces of fatty sub- stances are found in the ageing exodermis all entrance into cortex and stele of the substances used is blocked. 1. INTRODUCTION be On the structure of the Casparian strips many contradictory statements can found in the literature.It is often stated that the Casparian strips contain cutin, suberin, orsimilarfatty substances. Esau(1965), Scott-Russell & Barber(1960), Brouwer(1954, 1965), Priestley & North (1922), Priestley & Rhodes (1926) and van Wisselingh (1926) have stated that unsaturated fatty-acids are found in the strips. Frey-Wyssling (1959) doubted the function of these acids in the Casparian strips and suggested that the substances might be precursors of the substance later the inner surface of fatty which at a stage covers whole the endodermal cells. Kroemer (1903) and later Mylius (1913) and Ziegenspeck doubted in the (1921) the occurrence of fatty substances the Casparian strips as results of attemps to stain the strips with fat-colouring substances were negative. Warden (1935) has reported negative results with Sudan III and Sudan IV in Senecio vulgaris. -

Quantitative and Histomorphological Studies on Age-Correlated Changes in Canine and Porcine Hypophysis Lakshminarayana Das Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1971 Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis Lakshminarayana Das Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Structures Commons, and the Veterinary Anatomy Commons Recommended Citation Das, Lakshminarayana, "Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis" (1971). Retrospective Theses and Dissertations. 4873. https://lib.dr.iastate.edu/rtd/4873 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. 71-26,847 DAS, Lakshminarayana, 1936- QUANTITATIVE AND HISTOMORPHOLOGICAL STUDIES ON AGE-CORRELATED CHANGES IN CANINE AND PORCINE HYPOPHYSIS (VOLUMES I AND II). Iowa State University, Ph.D., 1971 Anatomy• University Microfilms, A XEROX Company, Ann Arbor. Michigan Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis py Lakshminarayana Das Volume 1 of 2 A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OP PHILOSOPHY Major Subject: -

Effects of Decalcified and Undecalcified Nacre of Freshwater Mollusk Hyriopsis Schlegeli on Mouse Osteoblasts (MC3T3-E1) to Induce Osteoid in Vitro
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Effects of decalcified and undecalcified nacre of freshwater mollusk Hyriopsis schlegeli on mouse osteoblasts (MC3T3-E1) to induce osteoid in vitro 著者 Su Nan-Chang, YAMAMOTO Gaku, KIMURA Yusuke, YOSHITAKE Kazusada journal or 滋賀医科大学雑誌 publication title volume 17 page range 63-71 year 2002-02 その他の言語のタイ マウスの骨芽細胞(MC3T3-E1)を用いた淡水真珠貝 トル Hyriopsis schlegeliの骨誘導に対する影響 : in vitroにおける脱灰と未脱灰粉の比較 マウス ノ コツガ サイボウ MC3T3-E1 ヲ モチイタ タンスイ シンジュガイ Hyriopsis schlegeli ノ ホネ ユウドウ ニ タイスル エイキョウ : in vitro ニ オケル ダクカイ ト ミダクカイコ ノ ヒ カク URL http://hdl.handle.net/10422/122 滋賀医大誌 17,63‐70,2002 Effects of Decalcified and Undecalcified Nacre of Freshwater Mollusk Hyriopsis schlegeli on Mouse Osteoblasts (MC3T3-E1) to Induce Osteoid in vitro Nan-CHANG Su, Gaku YAMAMOTO, Yusuke KIMURA, Kazusada YOSHITAKE Department of Oral & Maxillofacial Surgery, Shiga University of Medical Science Abstract: We tested the nacre of the freshwater mollusk Hyriopsis schlegeli for its ability to induce the os- teogenesis in the present study. Powdered EDTA-decalcified (0.3-1mm) and undecalcified nacre (300-425µm) were cultured with mouse osteoblasts (MC3T3-E1) for four weeks. Three experimental groups were com- pared in the present study. Medium for the culture was a combination of alpha-modified Eagle's medium, 10 % fetal bovine serum, and 1 % L-glutamine. The group A (n=10) applied undecalcified nacreous powder alone, whereas the group B (n=10) applied only decalcified nacreous powder. In group A and B, one grain of na- creous powder was placed in each dish.