Dynamic Interplay Between Pericytes and Endothelial Cells During Sprouting Angiogenesis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Cardiovascular System 9
Chapter Cardiovascular System 9 Learning Outcomes On completion of this chapter, you will be able to: 1. State the description and primary functions of the organs/structures of the car- diovascular system. 2. Explain the circulation of blood through the chambers of the heart. 3. Identify and locate the commonly used sites for taking a pulse. 4. Explain blood pressure. 5. Recognize terminology included in the ICD-10-CM. 6. Analyze, build, spell, and pronounce medical words. 7. Comprehend the drugs highlighted in this chapter. 8. Describe diagnostic and laboratory tests related to the cardiovascular system. 9. Identify and define selected abbreviations. 10. Apply your acquired knowledge of medical terms by successfully completing the Practical Application exercise. 255 Anatomy and Physiology The cardiovascular (CV) system, also called the circulatory system, circulates blood to all parts of the body by the action of the heart. This process provides the body’s cells with oxygen and nutritive ele- ments and removes waste materials and carbon dioxide. The heart, a muscular pump, is the central organ of the system. It beats approximately 100,000 times each day, pumping roughly 8,000 liters of blood, enough to fill about 8,500 quart-sized milk cartons. Arteries, veins, and capillaries comprise the network of vessels that transport blood (fluid consisting of blood cells and plasma) throughout the body. Blood flows through the heart, to the lungs, back to the heart, and on to the various body parts. Table 9.1 provides an at-a-glance look at the cardiovascular system. Figure 9.1 shows a schematic overview of the cardiovascular system. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -
Why Is Plasma Renin Activity Lower in Populations of African Origin?
Journal of Human Hypertension (2001) 15, 17–25 2001 Macmillan Publishers Ltd All rights reserved 0950-9240/01 $15.00 www.nature.com/jhh REVIEW ARTICLE Why is plasma renin activity lower in populations of African origin? GA Sagnella Blood Pressure Unit, St George’s Hospital Medical School, Cranmer Terrace, London SW17 ORE, UK Plasma renin activity is significantly lower in black the molecular level suggests that the lower PRA may people compared with whites independent of age and arise from gene variation in the renal epithelial sodium blood pressure status. The lower PRA appears to be due channel. The functional significance of the lower PRA in to a reduction in the rate of secretion of renin but the relation to the different pattern of cardiovascular and exact mechanistic events underlying such differences in renal disease between blacks and whites remains renin release between blacks and whites are still not unclear. Moreover, direct investigations of pre-treat- fully understood. Nevertheless, given the paramount ment renin status in hypertensive blacks in relation to importance of the renin-angiotensin system in the con- blood pressure response have demonstrated that the trol of sodium balance, a most likely explanation is that pre-treatment PRA is not a good index of subsequent the lower renin is a consequence of differences in renal blood pressure response to pharmacological treatment. sodium handling between blacks and whites. The lower Nevertheless, the blood pressure reduction to short PRA does not reflect differences in dietary sodium term sodium restriction is greater in blacks compared intake but the evidence available suggests that the low with whites and, in the black subjects, the greater PRA could be part of the corrective mechanisms reduction in blood pressure to sodium restriction designed to maintain sodium balance in the presence appears to be related, at least in part, to the decreased of an increased tendency for sodium retention in black responsiveness of the renin-angiotensin system. -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -
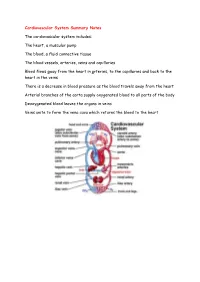
Cardiovascular System Summary Notes the Cardiovascular System
Cardiovascular System Summary Notes The cardiovascular system includes: The heart, a muscular pump The blood, a fluid connective tissue The blood vessels, arteries, veins and capillaries Blood flows away from the heart in arteries, to the capillaries and back to the heart in the veins There is a decrease in blood pressure as the blood travels away from the heart Arterial branches of the aorta supply oxygenated blood to all parts of the body Deoxygenated blood leaves the organs in veins Veins unite to form the vena cava which returns the blood to the heart Pulmonary System This is the route by which blood is circulated from the heart to the lungs and back to the heart again The pulmonary system is exceptional in that the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood Hepatic Portal Vein There is another exception in the circulatory system – the hepatic portal vein Veins normally carry blood from an organ back to the heart The hepatic portal vein carries blood from the capillary bed of the intestine to the capillary bed of the liver As a result, the liver has three blood vessels associated with it Arteries and Veins The central cavity of a blood vessel is called the lumen The lumen is lined with a thin layer of cells called the endothelium The composition of the vessel wall surrounding the endothelium is different in arteries, veins and capillaries Arteries carry blood away from the heart Arteries have a thick middle layer of smooth muscle They have an inner and outer layer of elastic fibres Elastic -

Blood Vessels
BLOOD VESSELS Blood vessels are how blood travels through the body. Whole blood is a fluid made up of red blood cells (erythrocytes), white blood cells (leukocytes), platelets (thrombocytes), and plasma. It supplies the body with oxygen. SUPERIOR AORTA (AORTIC ARCH) VEINS & VENA CAVA ARTERIES There are two basic types of blood vessels: veins and arteries. Veins carry blood back to the heart and arteries carry blood from the heart out to the rest of the body. Factoid! The smallest blood vessel is five micrometers wide. To put into perspective how small that is, a strand of hair is 17 micrometers wide! 2 BASIC (ARTERY) BLOOD VESSEL TUNICA EXTERNA TUNICA MEDIA (ELASTIC MEMBRANE) STRUCTURE TUNICA MEDIA (SMOOTH MUSCLE) Blood vessels have walls composed of TUNICA INTIMA three layers. (SUBENDOTHELIAL LAYER) The tunica externa is the outermost layer, primarily composed of stretchy collagen fibers. It also contains nerves. The tunica media is the middle layer. It contains smooth muscle and elastic fiber. TUNICA INTIMA (ELASTIC The tunica intima is the innermost layer. MEMBRANE) It contains endothelial cells, which TUNICA INTIMA manage substances passing in and out (ENDOTHELIUM) of the bloodstream. 3 VEINS Blood carries CO2 and waste into venules (super tiny veins). The venules empty into larger veins and these eventually empty into the heart. The walls of veins are not as thick as those of arteries. Some veins have flaps of tissue called valves in order to prevent backflow. Factoid! Valves are found mainly in veins of the limbs where gravity and blood pressure VALVE combine to make venous return more 4 difficult. -

Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury
BRIEF COMMUNICATION www.jasn.org Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury † ‡ ‡ ‡ Rafael Kramann,* Janewit Wongboonsin, § Monica Chang-Panesso, Flavia G. Machado, and ‡ Benjamin D. Humphreys *Renal Division, Brigham and Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts; †Division of Nephrology and Clinical Immunology, RWTH Aachen University Medical Faculty, RWTH Aachen University, Aachen, Germany; ‡Division of Nephrology, Department of Medicine, Washington University School of Medicine in St. Louis, St. Louis, Missouri; and §Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand ABSTRACT Peritubular capillary rarefaction is hypothesized to contribute to the increased risk of after AKI, and whether pericyte loss is future CKD after AKI. Here, we directly tested the role of Gli1+ kidney pericytes in the sufficient to induce peritubular capillary maintenance of peritubular capillary health, and the consequences of pericyte loss loss and altered morphology. during injury. Using bigenic Gli1-CreERt2; R26tdTomato reporter mice, we observed We genetically labeled pericytes using increased distance between Gli1+ pericytes and endothelial cells after AKI (mean6 Gli1-CreERt2 mice crossed against SEM: 3.360.1 mm before injury versus 12.560.2 mm after injury; P,0.001). Using a the R26tdTomato reporter mouse (Gt genetic ablation model, we asked whether pericyte loss alone is sufficient for capillary (ROSA)26Sortm9(CAF-tdTomato)Hze/J; destabilization. Ten days after pericyte ablation, we observed endothelial cell damage Figure 1A). After tamoxifen injection, mice by electron microscopy. Furthermore, pericyte loss led to significantly reduced capil- were subjected to severe unilateral ische- lary number at later time points (mean6SEM capillaries/high-power field: 67.664.7 in mia reperfusion injury (IRI; 28-minute control versus 44.164.8 at 56 days; P,0.05) and increased cross-sectional area (mean6 clamp). -

Fetal Descending Aorta/Umbilical Artery Flow Velocity Ratio in Normal Pregnancy at 36-40 Weeks of Gestational Age Riyadh W Alessawi1
American Journal of BioMedicine AJBM 2015; 3(10):674 - 685 doi:10.18081/2333-5106/015-10/674-685 Fetal descending aorta/umbilical artery flow velocity ratio in normal pregnancy at 36-40 Weeks of gestational age Riyadh W Alessawi1 Abstract Doppler velocimetry studies of placental and aortic circulation have gained a wide popularity as it can provide important information regarding fetal well-being and could be used to identify fetuses at risk of morbidity and mortality, thus providing an opportunity to improve fetal outcomes. Prospective longitudinal study conducted through the period from September 2011–July 2012, 125 women with normal pregnancy and uncomplicated fetal outcomes were recruited and subjected to Doppler velocimetry at different gestational ages, from 36 to 40 weeks. Of those, 15 women did not fulfill the protocol inclusion criteria and were not included. In the remaining 110 participants a follow up study of Fetal Doppler velocimetry of Ao and UA was performed at 36 – 40 weeks of gestation. Ao/UA RI: 1.48±0.26, 1.33±0.25, 1.37± 0.20, 1.28±0.07 and 1.39±0.45 respectively and the 95% confidence interval of the mean for five weeks 1.13-1.63. Ao/UA PI: 2.83±2.6, 1.94±0.82, 2.08±0.53, 1.81± 0.12 and 3.28±2.24 respectively. Ao/UA S/D: 2.14±0.72, 2.15±1.14, 1.75±0.61, 2.52±0.18 and 2.26±0.95. The data concluded that a nomogram of descending aorto-placental ratio Ao/UA, S/D, PI and RI of Iraqi obstetric population was established. -

SGLT2 Inhibition and Potential Renal Protection
The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto, MD Padua, Italy June 14, 2019 - Budapest, Hungary SGLT2 inhibition in CKD: Discussing the key questions and evidence Budapest, june 14 2019 The knowns and unknowns of SGLT2 inhibition in CKD Paola Fioretto Department of Medicine University of Padova, Italy 180 g of glucose filtered Glomerulus Proximal tubule Distal tubule Collecting duct each day S1 S2 Glucose filtration S3 SGLT2 SGLT1 90% 10% Glucose reabsorption Loop of Henle Up to ~ 90% of glucose ~ 10% of glucose Minimal is reabsorbed is reabsorbed glucose from the S1/S2 segments from the S3 segment excretion Possible mechanisms responsible for cardiovascular and renal protection with SGLT2 inhibition SGLT2 inhibition Glycosuria Natriuresis ↓Blood ↓Plasma Negative caloric balance ↑Uricosuria pressure ↑Tubuloglomerular volume feedback ↓Myocardial ↓HbA1c ↓ Afferent stretch ↓ ↓Plasma uric ↓Arterial ↓ arteriole acid stiffness ↑ constriction ↓Total body fat mass ↓Inflammation ↓Glucose toxicity ↓Epicardial fat ↓Intraglomerular hypertension ↓Ventricular ↓Hyperfiltration arrhythmias ↓Atherosclerosis Activation of ACE2 – Ang1/7 ↑Cardiac contractility ↓Inflammation No sympathetic nervous system activation ↓Fibrosis Cardiac and renal protection Heerspink HJ et al, Circulation 2016 Tonneijck et al, J Am Soc Nephrol 2017 Diabetic nephron Diabetic nephron with SGLT2 i Effects of SGLT2 i on afferent arteriole tone: in vivo studies with multiphoton microscope imaging techniques Kidokoro K et al, Circulation 2019 Effects of SGLT2 i -

The Dynamic Roles of Red Blood Cell in Microcirculation
Rochester Institute of Technology RIT Scholar Works Theses 7-15-2019 The Dynamic Roles of Red Blood Cell in Microcirculation Sitong Zhou [email protected] Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Zhou, Sitong, "The Dynamic Roles of Red Blood Cell in Microcirculation" (2019). Thesis. Rochester Institute of Technology. Accessed from This Dissertation is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. R.I.T The Dynamic Roles of Red Blood Cell in Microcirculation by Sitong Zhou A dissertation submitted in partial fulfillment of the requirements for the degree of Doctorate of Philosophy in Microsystems Engineering Microsystems Engineering Program Kate Gleason College of Engineering Rochester Institute of Technology Rochester, New York July 15th, 2019 1 The Dynamic Roles of Red Blood Cell in Microcirculation by Sitong Zhou Committee Approval: We, the undersigned committee members, certify that we have advised and/or supervised the candidate on the work described in this dissertation. We further certify that we have reviewed the dissertation manuscript and approve it in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Microsystems Engineering. ______________________________________________________________________________ Dr. Jiandi Wan Date Assistant Professor, Chemical Engineering ______________________________________________________________________________ -

Inferior Phrenic Artery, Variations in Origin and Clinical Implications – a Case Study
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) E-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 7, Issue 6 (Mar.- Apr. 2013), PP 46-48 www.iosrjournals.org Inferior Phrenic Artery, Variations in Origin and Clinical Implications – A Case Study 1 2 3 Dr.Anupama D, Dr.R.Lakshmi Prabha Subhash .Dr. B.S Suresh Assistant Professor. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Professor & HOD. Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Associate professor.Dept. Of Anatomy, SSMC. Tumkur.Karnataka.India Abstract:Variations in the branching pattern of abdominal aorta are quite common, knowledge of which is required to avoid complications during surgical interventions involving the posterior abdominal wall. Inferior Phrenic Arteries, the lateral aortic branches usually arise from Abdominal Aorta ,just above the level of celiac trunk. Occasionally they arise from a common aortic origin with celiac trunk, or from the celiac trunk itself or from the renal artery. This study describes the anomalous origin of this lateral or para aortic branches in the light of embryological and surgical basis. Knowledge of such variations has important clinical significance in abdominal operations like renal transplantation, laparoscopic surgery, and radiological procedures in the upper abdomen or invasive arterial procedures . Keywords: Abdominal Aorta, Celiac Trunk(Ct), Diaphragm, Inferior Phrenic Artery (Ipa), Retro Peritoneal, Renal Artery(Ra). I. Introduction The abdominal aorta begins from the level of 12th thoracic vertebra after passing through the Osseo aponeurotic hiatus of diaphragm. It courses downwards with Inferior vena cava to its right and terminates at the level of 4th lumbar vertebra by dividing in to two terminal branches.