The Effect of Bone and Ligament Morphology of Ankle Joint Loading in the Neutral Position
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Medial Lateral Malleolus
Acutrak 2® Headless Compression Screw System 4.7 mm and 5.5 mm Screws Supplemental Use Guide—Medial & Lateral Malleolus Acumed® is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve patient care. Acumed® Acutrak 2® Headless Compression Screw System—4.7 mm and 5.5 mm This guide is intended for supplemental use only and is not intended to be used as a stand-alone surgical technique. Reference the Acumed Acutrak 2 Headless Compression Screw System Surgical Technique (SPF00-02) for more information. Definition Indicates critical information about a potential serious outcome to the Warning patient or the user. Indicates instructions that must be followed in order to ensure the proper Caution use of the device. Note Indicates information requiring special attention. Acumed® Acutrak 2® Headless Compression System—Supplemental Use Guide—Medial & Lateral Malleolus Table of Contents System Features ...........................................2 Surgical Techniques ........................................ 4 Fibula Fracture (Weber A and B Fractures) Surgical Technique: Acutrak 2®—5.5 .......................4 Medial Malleolus Surgical Technique: Acutrak 2®—4.7 ......................10 Ordering Information ......................................16 Acumed® Acutrak 2® Headless Compression System—Supplemental Use Guide—Medial & Lateral Malleolus System Features Headless screw design is intended to minimize soft tissue irritation D Acutrak 2 Screws Diameter -

Assessment, Management and Decision Making in the Treatment Of
Pediatric Ankle Fractures Anthony I. Riccio, MD Texas Scottish Rite Hospital for Children Update 07/2016 Pediatric Ankle Fractures The Ankle is the 2nd most Common Site of Physeal Injury in Children 10-25% of all Physeal Injuries Occur About the Ankle Pediatric Ankle Fractures Primary Concerns Are: • Anatomic Restoration of Articular Surface • Restoration of Symmetric Ankle Mortise • Preservation of Physeal Growth • Minimize Iatrogenic Physeal Injury • Avoid Fixation Across Physis in Younger Children Salter Harris Classification Prognosis and Treatment of Pediatric Ankle Fractures is Often Dictated by the Salter Harris Classification of Physeal Fractures Type I and II Fractures: Often Amenable to Closed Tx / Lower Risk of Physeal Arrest Type III and IV: More Likely to Require Operative Tx / Higher Risk of Physeal Arrest Herring JA, ed. Tachdjian’s Pediatric Orthopaedics, 5th Ed. 2014. Elsevier. Philadelphia, PA. ISOLATED DISTAL FIBULA FRACTURES Distal Fibula Fractures • The Physis is Weaker than the Lateral Ankle Ligaments – Children Often Fracture the Distal Fibula but…. – …ligamentous Injuries are Not Uncommon • Mechanism of Injury = Inversion of a Supinated Foot • SH I and II Fractures are Most Common – SH I Fractures: Average Age = 10 Years – SH II Fractures: Average Age = 12 Years Distal Fibula Fractures Lateral Ankle Tenderness SH I Distal Fibula Fracture vs. Lateral Ligamentous Injury (Sprain) Distal Fibula Fractures • Sankar et al (JPO 2008) – 37 Children – All with Open Physes, Lateral Ankle Tenderness + Normal Films – 18%: Periosteal -

Free Vascularized Fibula Graft with Femoral Allograft Sleeve for Lumbar Spine Defects After Spondylectomy of Malignant Tumors Acasereport
1 COPYRIGHT Ó 2020 BY THE JOURNAL OF BONE AND JOINT SURGERY,INCORPORATED Free Vascularized Fibula Graft with Femoral Allograft Sleeve for Lumbar Spine Defects After Spondylectomy of Malignant Tumors ACaseReport Michiel E.R. Bongers, MD, John H. Shin, MD, Sunita D. Srivastava, MD, Christopher R. Morse, MD, Sang-Gil Lee, MD, and Joseph H. Schwab, MD, MS Investigation performed at Massachusetts General Hospital, Boston, Massachusetts Abstract Case: We present a 65-year-old man with an L4 conventional chordoma. Total en bloc spondylectomy (TES) of the involved vertebral bodies and surrounding soft tissues with reconstruction of the spine using a free vascularized fibula autograft (FVFG) is a proven technique, limiting complications and recurrence. However, graft fracture has occurred only in the lumbar spine in our institutional cases. We used a technique in our patient to ensure extra stability and support, with the addition of a femoral allograft sleeve encasing the FVFG. Conclusions: Our technique for the reconstruction of the lumbar spine after TES of primary malignant spinal disease using a femoral allograft sleeve encasing the FVFG is viable to consider. he treatment of primary malignant neoplasms of the spine mended a magnetic resonance imaging (MRI), but the request currently mainly relies on surgery, often in conjunction with was denied by the insurance company, and the patient T 1-3 radiotherapy .Totalen bloc spondylectomy (TES) is a widely underwent a course of physical therapy with no benefitand accepted surgical technique and has lower reported recurrence rates progression of back pain and radiculopathy. Four months compared with patients who undergo intralesional surgery3,4. -

Common Stress Fractures BRENT W
COVER ARTICLE PRACTICAL THERAPEUTICS Common Stress Fractures BRENT W. SANDERLIN, LCDR, MC, USNR, Naval Branch Medical Clinic, Fort Worth, Texas ROBERT F. RASPA, CAPT, MC, USN, Naval Hospital Jacksonville, Jacksonville, Florida Lower extremity stress fractures are common injuries most often associated with partic- ipation in sports involving running, jumping, or repetitive stress. The initial diagnosis can be made by identifying localized bone pain that increases with weight bearing or repet- itive use. Plain film radiographs are frequently unrevealing. Confirmation of a stress frac- ture is best made using triple phase nuclear medicine bone scan or magnetic resonance imaging. Prevention of stress fractures is most effectively accomplished by increasing the level of exercise slowly, adequately warming up and stretching before exercise, and using cushioned insoles and appropriate footwear. Treatment involves rest of the injured bone, followed by a gradual return to the sport once free of pain. Recent evidence sup- ports the use of air splinting to reduce pain and decrease the time until return to full par- ticipation or intensity of exercise. (Am Fam Physician 2003;68:1527-32. Copyright© 2003 American Academy of Family Physicians) tress fractures are among the involving repetitive use of the arms, such most common sports injuries as baseball or tennis. Stress fractures of and are frequently managed the ribs occur in sports such as rowing. by family physicians. A stress Upper extremity and rib stress fractures fracture should be suspected in are far less common than lower extremity Sany patient presenting with localized stress fractures.1 bone or periosteal pain, especially if he or she recently started an exercise program Etiology and Pathophysiology or increased the intensity of exercise. -
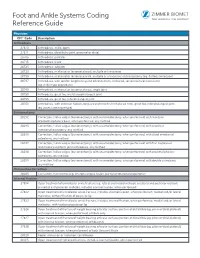
Foot and Ankle Systems Coding Reference Guide
Foot and Ankle Systems Coding Reference Guide Physician CPT® Code Description Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis; triple 28725 Arthrodesis; subtalar 28730 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse 28735 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse; with osteotomy (eg, flatfoot correction) 28737 Arthrodesis, with tendon lengthening and advancement, midtarsal, tarsal navicular-cuneiform (eg, miller type procedure) 28740 Arthrodesis, midtarsal or tarsometatarsal, single joint 28750 Arthrodesis, great toe; metatarsophalangeal joint 28755 Arthrodesis, great toe; interphalangeal joint 28760 Arthrodesis, with extensor hallucis longus transfer to first metatarsal neck, great toe, interphalangeal joint (eg, jones type procedure) Bunionectomy 28292 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with resection of proximal phalanx base, when performed, any method 28295 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with proximal metatarsal osteotomy, any method 28296 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with distal metatarsal osteotomy, any method 28297 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with first metatarsal and medial cuneiform joint arthrodesis, any method 28298 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with -
Bones of the Upper and Lower Limb Musculoskeletal Block - Lecture 1
Bones of the Upper and Lower limb Musculoskeletal Block - Lecture 1 Objective: Classify the bones of the three regions of the lower limb (Thigh, leg and foot). Memorize the main features of the – Bones of the thigh (femur & patella) – Bones of the leg (tibia & Fibula) – Bones of the foot (tarsals, metatarsals and phalanges) Recognize the side of the bone. Color index: Important In male’s slides only In female’s slides only Extra information, explanation Editing file Click here for Contact us: useful videos [email protected] Please make sure that you’re familiar with these terms Terms Meaning Example Ridge The long and narrow upper edge, angle, or crest of something The supracondylar ridges (in the distal part of the humerus) Notch An indentation, (incision) on an edge or surface The trochlear notch (in the proximal part of the ulna) Tubercles A nodule or a small rounded projection on the bone (Dorsal tubercle in the distal part of the radius) Fossa A hollow place (The Notch is not complete but the fossa is Subscapular fossa (in the concave part of complete and both of them act as the lock of the joint the scapula) Tuberosity A large prominence on a bone usually serving for Deltoid tuberosity (in the humorous) and it the attachment of muscles or ligaments ( is a bigger projection connects the deltoid muscle than the Tubercle ) Processes A V-shaped indentation (act as the key of the joint) Coracoid process ( in the scapula ) Groove A channel, a long narrow depression sure Spiral (Radial) groove (in the posterior aspect of (the humerus -

Stress Fractures in the Foot and Ankle of Athletes Fratura Por Estresse No Pé E Tornozelo De Atletas Authors: Asano LYJ, Duarte Jr
GUIDELINES IN FOCUS ASANO LYJ ET al. Stress fractures in the foot and ankle of athletes FRATURA POR ESTRESSE NO PÉ E TORNOZELO DE ATLETAS Authors: Asano LYJ, Duarte Jr. A, Silva APS http://dx.doi.org/10.1590/1806-9282.60.06.006 The Guidelines Project, an initiative of the Brazilian Medical Association, aims to combine information from the medical field in order to standar- dize procedures to assist the reasoning and decision-making of doctors. The information provided through this project must be assessed and criticized by the physician responsible for the conduct that will be adopted, de- pending on the conditions and the clinical status of each patient. DESCRIPTION OF THE EVIDENCE COLLECTION INTRODUCTION METHOD Stress fractures were described for the first time in 1855 To develop this guideline, the Medline electronic databa- by Breihaupt among soldiers reporting plantar pain and se (1966 to 2012) was consulted via PubMed, as a primary edema following long marches.1 For athletes, the first cli- base. The search for evidence came from actual clinical nical description was given by Devas in 1958, based so- scenarios and used keywords (MeSH terms) grouped in lely on the results of simple X-rays.2 Stress injuries are the following syntax: “Stress fractures”, “Foot”, “Ankle”, common among athletes and military recruits, accoun- “Athletes”, “Professional”, “Military recruit”, “Immobili- ting for approximately 10% of all orthopedic injuries.3 zation”, “Physiotherapy”, “Rest”, “Rehabilitation”, “Con- It is defined as a solution for partial or complete con- ventional treatment”, “Surgery treatment”. The articles tinuity of a bone as a result of excessive or repeated loads, were selected by orthopedic specialists after critical eva- at submaximal intensity, resulting in greater reabsorp- luation of the strength of scientific evidence, and publi- tion faced with an insufficient formation of bone tissue.1 cations of greatest strength were used for recommenda- Although stress fractures may affect all types of bone tion. -

Bones of the Lower Limb
Bones of the Lower Limb Lecture 2 Please check our Editing File. َ َّ َ َ وََمنْ َيتوَكلْ عَلى اَّّللْه ْفوُهوَْ } هذا العمل ﻻ يغني عن المصدر اﻷساسي للمذاكرة {حَس بووهْ Objectives ● Classify the bones of the three regions of the lower limb (thigh, leg and foot). ● Differentiate the bones of the lower limb from the bones of the upper limb. ● Memorize the main features of the Bones of the thigh (femur & patella) Bones of the leg (tibia & Fibula). Bones of the foot (tarsals, metatarsals and phalanges) ● Recognize the side of the bone ● Text in BLUE was found only in the boys’ slides ● Text in PINK was found only in the girls’ slides ● Text in RED is considered important ● Text in GREY is considered extra notes Bones of Thigh (Femur and Patella) ❖ Femur: ➢ Articulates above with acetabulum of hip bone to form the hip joint. Thigh ➢ Articulates below with tibia and patella to form the knee joint. (The Fibula have no part in Knee joint) Knee ❖ In thigh region 1 bone ❖ In leg region 2 bones Leg ❖ In foot region 26 bones ❖ (Long bone) Any bone has two ends and a shaft Foot ❖ The only short bones are: Carpals and Tarsals ❖ (Sesamoid) a bone within the tendon of a muscle Bones of Thigh (Femur and Patella) Femur ❖ consists of : ➢ Upper end ➢ Shaft ➢ Lower end Upper End Of The Femur 1.Head: -It articulates with acetabulum of hip bone to form hip joint. -Has a depression in the center (Fovea capitis) for the attachment of ligament of the head of femur. -

Femur - Patella - Tibia - Fibula - Soft Tissue: Depending on the Degree of Exposure
DISPLAYED STRUCTURES - Femur - Patella - Tibia - Fibula - Soft tissue: depending on the degree of exposure VIEWS Lateromedial and caudolateral view craneomedial oblique (30 °) The chassis is positioned medially in the region of the stifle flow and dorsal as possible, with caution because it is a sensitive area on the horse. The beam is directed parallel to the ground laterally and centered on the patella. Depending on the temperament of the horse sometimes can not be fully appreciated in this view of the distal femur and patella. The side view oblique (caudolateral oblique craneomedial) permte appreciate osteochondral lesions of the medial condyle and the lateral edge of the trochlea on the distal femur. View caudocranial The chassis is positioned superiorly in the area of the stifle and the beam is focused on the concave region is seen in the soft tissues of the joint. Sometimes it is useful to perform two projections with varying degrees of exposure in order to appreciate all structures. View proximodistal ("skyline") Depending on the temperament of the horse can do this by extending or flexing view the tarsus. The chassis is held pressed against the proximal tibia and cranially and the beam is directed vertically proximal and lateral to the lumbar region. We extend the foot in maximum amplitude caudally leading him, and put the frame parallel to the ground supporting it the joint. The beam perpendicular to the ground 70 cm. dorsal to the stifle. DIAGNOSTIC UTILITY • Osteochondrosis • In the area of the lateral trochlear ridge of the femur and the patella articular surface is where we find most often an osteochondral defect with cartilage separation. -
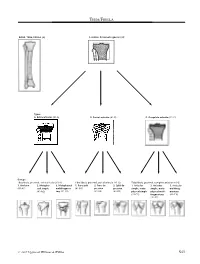
Tibia/Fibula
TIBIA/FIBULA BONE: TIBIA/FIBULA (4) Location: Proximal segment (41) Types: A. Extra-articular (41-A) B. Partial articular (41-B) C. Complete articular (41-C) Groups: Tibia/fibula, proximal, extra-articular (41-A) Tibia/fibula, proximal, partial articular (41-B) Tibia/fibula, proximal, complete articular (41-C) 1. Avulsion 2. Metaphy- 3. Metaphyseal 1. Pure split 2. Pure de- 3. Split de- 1. Articular 2. Articular 3. Articular (41-A1) seal simple multifragmen- (41-B1) pression pression simple, meta- simple, meta- multifrag- (41-A2) tary (41-A3) (41-B2) (41-B3) physeal simple physeal multi- mentary (41-C1) fragmentary (41-C3) (41-C2) © 2007 Lippincott Williams & Wilkins S43 Tibia/Fibula J Orthop Trauma • Volume 21, Number 10 Supplement, November/December 2007 Subgroups and Qualifications: Tibia/fibula, proximal, extra-articular, avulsion (41-A1) 1. Of fibular head (41-A1.1) 2. Of tibial tuberosity (41-A1.2) 3. Of cruciate insertion (41-A1.3) (1) anterior (2) posterior A1 Tibia/fibula, proximal, extra-articular, simple metaphysis (41-A2) 1. Oblique in frontal plane (41-A2.1) 2. Oblique in sagittal plane (41-A2.2) 3. Transverse (41-A2.3) A2 Tibia/fibula, proximal, extra-articular, multifragmentary metaphysis (41-A3) 1. Intact wedge (41-A3.1) 2. Fragmented wedge (41-A3.2) 3. Complex (41-A3.3) (1) lateral (1) lateral (1) slightly displaced (2) medial (2) medial (2) significantly displaced A3 S44 © 2007 Lippincott Williams & Wilkins J Orthop Trauma • Volume 21, Number 10 Supplement, November/December 2007 Tibia/Fibula Tibia/fibula, proximal, partial articular, split (41-B1) 1. Of lateral surface (41-B1.1) 2. -

Bone Diagram.Pub
Bone Diagram Forehead The common name of (Frontal bone) each bone is listed first, Nose bones with the scientific name (Nasals) given in parenthesis. Cheek bone (Zygoma) Upper jaw (Maxilla) Lower jaw (Mandible) Collar bone (Clavicle) Breast bone (Sternum) Upper arm bone (Humerus) Lower arm bone (Ulna) Lower arm bone (Radius) Thigh bone (Femur) Kneecap (Patella) Shin bone (Tibia) Calf bone (Fibula) Ankle bones (Tarsals) Foot bones (Metatarsals) Toe bones (Phalanges) Skull Side of skull (Cranium) (Parietal bone) Back of skull Did you know? (Occipital bone) When you are a baby you Temple have more than 300 (Temporal bone) bones. By the time you are an adult you only Backbone Neck vertebrae (7) have 206 bones, because (Spine) (Cervical vertebrae) some of your bones join together as you grow! Shoulder blade (Scapula) Chest vertebrae (12) (Thoracic vertebrae) Ribs (12 pairs) Lower back vertebrae (5) (Lumbar vertebrae) Fused vertebrae (5) (Sacrum) Pelvic bones (Ilium) (Pubis) (Ischium) Wrist bones (Carpals) Hand bones (Metacarpals) Finger bones Bones are important! (Phalanges) They hold up your body, and along with your muscles, keep you moving. Without your bones, you’d just be one big blob! To be able to grow, strong bones needs lots of calcium and weight-bearing physical activity. Heel bone (Calcaneus) University of Washington PKU Clinic CHDD - Box 357920, Seattle, WA 98195 (206) 685-3015, Toll Free in Washington State 877-685-3015 http://depts.washington.edu/pku . -

Calcaneal Autografts: Indications, Technique, and Complications
CHAPTER 3 4 CALCANEAL AUTOGRAFTS: INDICATIONS, TECHNIQUE, AND COMPLICATIONS Andrea D. Cass, DPM INTRODUCTION podiatric surgeon in certain geographic areas or may require an orthopedic surgeon. Patient must be non-weight bearing When it comes to using bone grafts, everyone knows that for at least six weeks following harvesting graft from this site. autografts are superior to other types of bone and bone The distal tibia is a great place to obtain cancellous bone or substitutes. In foot and ankle surgery, autografts are not a cortical strut. This site has no effect on weight bearing, but always necessary. Bone voids are often filled with allogenic there is limited quantity of bone that can be harvested. cortical or cancellous bone, demineralized bone matrix, or The fibula is another good place to harvest autogenous bone. other commonly-accepted substitutes. In certain patients, The middle third of the fibula can be safely used. Some bone healing may be a concern and autograft is preferred to people advocate leaving the medial cortex intact for augment healing. This includes patients with co-morbidities regeneration, while others leave only the periosteum intact. such as diabetes mellitus, smoking, and increased age. This is a great source of cortical bone, but the dissection in Autografts can be harvested from many sites in the body this area is difficult due to the peroneal muscles and the including the anterior and posterior iliac crest, proximal tibia, common peroneal nerve. distal tibia, and the calcaneus. The iIliac crest is a great source The calcaneus is a great source of autogenous bone and for large cortical, corticocancellous, and cancellous bone.