A VISCOSITY PRIMER: VISCOSITY DEFINITIONS Viscosity Absolute
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Viscosity of Gases References
VISCOSITY OF GASES Marcia L. Huber and Allan H. Harvey The following table gives the viscosity of some common gases generally less than 2% . Uncertainties for the viscosities of gases in as a function of temperature . Unless otherwise noted, the viscosity this table are generally less than 3%; uncertainty information on values refer to a pressure of 100 kPa (1 bar) . The notation P = 0 specific fluids can be found in the references . Viscosity is given in indicates that the low-pressure limiting value is given . The dif- units of μPa s; note that 1 μPa s = 10–5 poise . Substances are listed ference between the viscosity at 100 kPa and the limiting value is in the modified Hill order (see Introduction) . Viscosity in μPa s 100 K 200 K 300 K 400 K 500 K 600 K Ref. Air 7 .1 13 .3 18 .5 23 .1 27 .1 30 .8 1 Ar Argon (P = 0) 8 .1 15 .9 22 .7 28 .6 33 .9 38 .8 2, 3*, 4* BF3 Boron trifluoride 12 .3 17 .1 21 .7 26 .1 30 .2 5 ClH Hydrogen chloride 14 .6 19 .7 24 .3 5 F6S Sulfur hexafluoride (P = 0) 15 .3 19 .7 23 .8 27 .6 6 H2 Normal hydrogen (P = 0) 4 .1 6 .8 8 .9 10 .9 12 .8 14 .5 3*, 7 D2 Deuterium (P = 0) 5 .9 9 .6 12 .6 15 .4 17 .9 20 .3 8 H2O Water (P = 0) 9 .8 13 .4 17 .3 21 .4 9 D2O Deuterium oxide (P = 0) 10 .2 13 .7 17 .8 22 .0 10 H2S Hydrogen sulfide 12 .5 16 .9 21 .2 25 .4 11 H3N Ammonia 10 .2 14 .0 17 .9 21 .7 12 He Helium (P = 0) 9 .6 15 .1 19 .9 24 .3 28 .3 32 .2 13 Kr Krypton (P = 0) 17 .4 25 .5 32 .9 39 .6 45 .8 14 NO Nitric oxide 13 .8 19 .2 23 .8 28 .0 31 .9 5 N2 Nitrogen 7 .0 12 .9 17 .9 22 .2 26 .1 29 .6 1, 15* N2O Nitrous -

On Nonlinear Strain Theory for a Viscoelastic Material Model and Its Implications for Calving of Ice Shelves
Journal of Glaciology (2019), 65(250) 212–224 doi: 10.1017/jog.2018.107 © The Author(s) 2019. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is unaltered and is properly cited. The written permission of Cambridge University Press must be obtained for commercial re- use or in order to create a derivative work. On nonlinear strain theory for a viscoelastic material model and its implications for calving of ice shelves JULIA CHRISTMANN,1,2 RALF MÜLLER,2 ANGELIKA HUMBERT1,3 1Division of Geosciences/Glaciology, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany 2Institute of Applied Mechanics, University of Kaiserslautern, Kaiserslautern, Germany 3Division of Geosciences, University of Bremen, Bremen, Germany Correspondence: Julia Christmann <[email protected]> ABSTRACT. In the current ice-sheet models calving of ice shelves is based on phenomenological approaches. To obtain physics-based calving criteria, a viscoelastic Maxwell model is required account- ing for short-term elastic and long-term viscous deformation. On timescales of months to years between calving events, as well as on long timescales with several subsequent iceberg break-offs, deformations are no longer small and linearized strain measures cannot be used. We present a finite deformation framework of viscoelasticity and extend this model by a nonlinear Glen-type viscosity. A finite element implementation is used to compute stress and strain states in the vicinity of the ice-shelf calving front. -

Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems
NfST Nisr National institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 946 Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems Vincent A. Hackley and Chiara F. Ferraris rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Application Note to the Field Pumping Non-Newtonian Fluids with Liquiflo Gear Pumps
Pumping Non-Newtonian Fluids Application Note to the Field with Liquiflo Gear Pumps Application Note Number: 0104-2 Date: April 10, 2001; Revised Jan. 2016 Newtonian vs. non-Newtonian Fluids: Fluids fall into one of two categories: Newtonian or non-Newtonian. A Newtonian fluid has a constant viscosity at a particular temperature and pressure and is independent of shear rate. A non-Newtonian fluid has viscosity that varies with shear rate. The apparent viscosity is a measure of the resistance to flow of a non-Newtonian fluid at a given temperature, pressure and shear rate. Newton’s Law states that shear stress () is equal the dynamic viscosity () multiplied by the shear rate (): = . A fluid which obeys this relationship, where is constant, is called a Newtonian fluid. Therefore, for a Newtonian fluid, shear stress is directly proportional to shear rate. If however, varies as a function of shear rate, the fluid is non-Newtonian. In the SI system, the unit of shear stress is pascals (Pa = N/m2), the unit of shear rate is hertz or reciprocal seconds (Hz = 1/s), and the unit of dynamic viscosity is pascal-seconds (Pa-s). In the cgs system, the unit of shear stress is dynes per square centimeter (dyn/cm2), the unit of shear rate is again hertz or reciprocal seconds, and the unit of dynamic viscosity is poises (P = dyn-s-cm-2). To convert the viscosity units from one system to another, the following relationship is used: 1 cP = 1 mPa-s. Pump shaft speed is normally measured in RPM (rev/min). -

James Clerk Maxwell
Conteúdo Páginas Henry Cavendish 1 Jacques Charles 2 James Clerk Maxwell 3 James Prescott Joule 5 James Watt 7 Jean-Léonard-Marie Poiseuille 8 Johann Heinrich Lambert 10 Johann Jakob Balmer 12 Johannes Robert Rydberg 13 John Strutt 14 Michael Faraday 16 Referências Fontes e Editores da Página 18 Fontes, Licenças e Editores da Imagem 19 Licenças das páginas Licença 20 Henry Cavendish 1 Henry Cavendish Referência : Ribeiro, D. (2014), WikiCiências, 5(09):0811 Autor: Daniel Ribeiro Editor: Eduardo Lage Henry Cavendish (1731 – 1810) foi um físico e químico que investigou isoladamente de acordo com a tradição de Sir Isaac Newton. Cavendish entrou no seminário em 1742 e frequentou a Universidade de Cambridge (1749 – 1753) sem se graduar em nenhum curso. Mesmo antes de ter herdado uma fortuna, a maior parte das suas despesas eram gastas em montagem experimental e livros. Em 1760, foi eleito Fellow da Royal Society e, em 1803, um dos dezoito associados estrangeiros do Institut de France. Entre outras investigações e descobertas de Cavendish, a maior ocorreu em 1781, quando compreendeu que a água é uma substância composta por hidrogénio e oxigénio, uma reformulação da opinião de há milénios de que a água é um elemento químico básico. O químico inglês John Warltire (1725/6 – 1810) realizou uma Figura 1 Henry Cavendish (1731 – 1810). experiência em que explodiu uma mistura de ar e hidrogénio, descobrindo que a massa dos gases residuais era menor do que a da mistura original. Ele atribuiu a perda de massa ao calor emitido na reação. Cavendish concluiu que algum erro substancial estava envolvido visto que não acreditava que, dentro da teoria do calórico, o calor tivesse massa suficiente, à escala em análise. -

Investigations of Liquid Steel Viscosity and Its Impact As the Initial Parameter on Modeling of the Steel Flow Through the Tundish
materials Article Investigations of Liquid Steel Viscosity and Its Impact as the Initial Parameter on Modeling of the Steel Flow through the Tundish Marta Sl˛ezak´ 1,* and Marek Warzecha 2 1 Department of Ferrous Metallurgy, Faculty of Metals Engineering and Industrial Computer Science, AGH University of Science and Technology, Al. Mickiewicza 30, 30-059 Kraków, Poland 2 Department of Metallurgy and Metal Technology, Faculty of Production Engineering and Materials Technology, Cz˛estochowaUniversity of Technology, Al. Armii Krajowej 19, 42-201 Cz˛estochowa,Poland; [email protected] * Correspondence: [email protected] Received: 14 September 2020; Accepted: 5 November 2020; Published: 7 November 2020 Abstract: The paper presents research carried out to experimentally determine the dynamic viscosity of selected iron solutions. A high temperature rheometer with an air bearing was used for the tests, and ANSYS Fluent commercial software was used for numerical simulations. The experimental results obtained are, on average, lower by half than the values of the dynamic viscosity coefficient of liquid steel adopted during fluid flow modeling. Numerical simulations were carried out, taking into account the viscosity standard adopted for most numerical calculations and the average value of the obtained experimental dynamic viscosity of the analyzed iron solutions. Both qualitative and quantitative analysis showed differences in the flow structure of liquid steel in the tundish, in particular in the predicted values and the velocity profile distribution. However, these differences are not significant. In addition, the work analyzed two different rheological models—including one of our own—to describe the dynamic viscosity of liquid steel, so that in the future, the experimental stage could be replaced by calculating the value of the dynamic viscosity coefficient of liquid steel using one equation. -

S.I. and Cgs Units
Some Notes on SI vs. cgs Units by Jason Harlow Last updated Feb. 8, 2011 by Jason Harlow. Introduction Within “The Metric System”, there are actually two separate self-consistent systems. One is the Systme International or SI system, which uses Metres, Kilograms and Seconds for length, mass and time. For this reason it is sometimes called the MKS system. The other system uses centimetres, grams and seconds for length, mass and time. It is most often called the cgs system, and sometimes it is called the Gaussian system or the electrostatic system. Each system has its own set of derived units for force, energy, electric current, etc. Surprisingly, there are important differences in the basic equations of electrodynamics depending on which system you are using! Important textbooks such as Classical Electrodynamics 3e by J.D. Jackson ©1998 by Wiley and Classical Electrodynamics 2e by Hans C. Ohanian ©2006 by Jones & Bartlett use the cgs system in all their presentation and derivations of basic electric and magnetic equations. I think many theorists prefer this system because the equations look “cleaner”. Introduction to Electrodynamics 3e by David J. Griffiths ©1999 by Benjamin Cummings uses the SI system. Here are some examples of units you may encounter, the relevant facts about them, and how they relate to the SI and cgs systems: Force The SI unit for force comes from Newton’s 2nd law, F = ma, and is the Newton or N. 1 N = 1 kg·m/s2. The cgs unit for force comes from the same equation and is called the dyne, or dyn. -
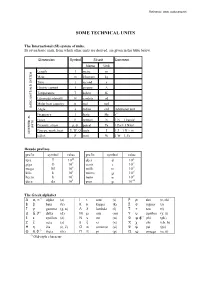
Technical Units
Reference: www. rockmass.net 620(7(&+1,&$/81,76 7KH,QWHUQDWLRQDO 6, V\VWHPRIXQLWV Its seven basic units, from which other units are derived, are given in the table below. Dimension Symbol SI unit Comment Name Unit Length l metre m Mass m kilogram kg Time t second s Electric current I ampere A Temperature T kelvin K Luminous intensity Iv candela cd Molar heat capacity n mol mol BASIC UNITS IN SI SYSTEM Angle a radian rad Additional unit Frequency f hertz Hz s-1 Force F newton N 1 N = 1 kgm/s2 Pressure, stress p, σ, pascal Pa 1 Pa = 1 N/m² UNITS Energy, work, heat E, W, Q joule J 1 J = 1 N × m DIVERTED SI- Effect P watt W 1 W = 1 J/s 'HFDGHSUHIL[HV prefix symbol value prefix symbol value tera T 1012 deci d 10-1 giga G 109 centi c 10-2 mega M 106 milli m 10-3 kilo k 103 micro µ 10-6 hecto h 102 nano n 10-9 deca da 101 pico p 10-12 7KH*UHHNDOSKDEHW Α α, ∝ ∗) alpha (a) Ι ι iota (i) Ρ ρ rho (r, rh) Β β beta (b) Κ κ kappa (k) Σ σ sigma (s) Γ γ gamma (g, n) Λ λ lambda (l) Τ τ tau (t) ∆ δ, ∂ ∗) delta (d) Μ µ mu (m) Υ υ upsilon (y, u) Ε ε epsilon (e) Ν ν nu (n) Φ φ, ϕ*) phi (ph) Ζ ζ zeta (z) Ξ ξ xi (x) X χ chi (ch, h) Η η ëta (e, ë) Ο ο omicron (o) Ψ ψ psi (ps) Θ θ, ϑ ∗) theta (th) Π π pi (p) Ω ω omega (o, õ) *) Old-style character 2 9DULRXVXQLWVDQGFRQYHUVLRQV 'LPHQVLRQ 1DPH 6\PERO 8QLW &RQYHUVLRQ Time minute min s 1 h = 60 s hour h h 1 h = 60 min day day day 1 day = 24 h week wk wk 1 wk = 7 days year yr yr 1 yr = 365.242 days = 52.17 wk Length Angstrom A m 1 A = 10-10 m nautical mile n mile 1 n mile = 1852 m wave length λ m Force load G (P,W) N 1 kp = 9.807 N 2 o o 2 gravity g N 1 gn = 9.80665 m/s (exact, at 45 N; at 60 N g = 9.82 m/s ) atmosphere atm 1 atm Modulus elasticity modulus E Pa 1 kPa = 0.1 N/cm = 0.102 ton/m2 deformation modulus G Pa 1 MPa = 10,2 kg/cm2 shear modulus K Pa Density mass density ρ kg/m3 force density γ kN/m3 Flow Lugeon Lugeon l/min/m 1 Lugeon = approx. -

DIMENSIONS and UNITS to Get the Value of a Quantity in Gaussian Units, Multiply the Value Ex- Pressed in SI Units by the Conversion Factor
DIMENSIONS AND UNITS To get the value of a quantity in Gaussian units, multiply the value ex- pressed in SI units by the conversion factor. Multiples of 3 intheconversion factors result from approximating the speed of light c =2.9979 1010 cm/sec × 3 1010 cm/sec. ≈ × Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units t2q2 Capacitance C l farad 9 1011 cm ml2 × m1/2l3/2 Charge q q coulomb 3 109 statcoulomb t × q m1/2 Charge ρ coulomb 3 103 statcoulomb 3 3/2 density l l t /m3 × /cm3 tq2 l Conductance siemens 9 1011 cm/sec ml2 t × 2 tq 1 9 1 Conductivity σ siemens 9 10 sec− 3 ml t /m × q m1/2l3/2 Current I,i ampere 3 109 statampere t t2 × q m1/2 Current J, j ampere 3 105 statampere 2 1/2 2 density l t l t /m2 × /cm2 m m 3 3 3 Density ρ kg/m 10− g/cm l3 l3 q m1/2 Displacement D coulomb 12π 105 statcoulomb l2 l1/2t /m2 × /cm2 1/2 ml m 1 4 Electric field E volt/m 10− statvolt/cm t2q l1/2t 3 × 2 1/2 1/2 ml m l 1 2 Electro- , volt 10− statvolt 2 motance EmfE t q t 3 × ml2 ml2 Energy U, W joule 107 erg t2 t2 m m Energy w, ϵ joule/m3 10 erg/cm3 2 2 density lt lt 10 Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units ml ml Force F newton 105 dyne t2 t2 1 1 Frequency f, ν hertz 1 hertz t t 2 ml t 1 11 Impedance Z ohm 10− sec/cm tq2 l 9 × 2 2 ml t 1 11 2 Inductance L henry 10− sec /cm q2 l 9 × Length l l l meter (m) 102 centimeter (cm) 1/2 q m 3 Magnetic H ampere– 4π 10− oersted 1/2 intensity lt l t turn/m × ml2 m1/2l3/2 Magnetic flux Φ weber 108 maxwell tq t m m1/2 Magnetic -

Equation of Motion for Viscous Fluids
1 2.25 Equation of Motion for Viscous Fluids Ain A. Sonin Department of Mechanical Engineering Massachusetts Institute of Technology Cambridge, Massachusetts 02139 2001 (8th edition) Contents 1. Surface Stress …………………………………………………………. 2 2. The Stress Tensor ……………………………………………………… 3 3. Symmetry of the Stress Tensor …………………………………………8 4. Equation of Motion in terms of the Stress Tensor ………………………11 5. Stress Tensor for Newtonian Fluids …………………………………… 13 The shear stresses and ordinary viscosity …………………………. 14 The normal stresses ……………………………………………….. 15 General form of the stress tensor; the second viscosity …………… 20 6. The Navier-Stokes Equation …………………………………………… 25 7. Boundary Conditions ………………………………………………….. 26 Appendix A: Viscous Flow Equations in Cylindrical Coordinates ………… 28 ã Ain A. Sonin 2001 2 1 Surface Stress So far we have been dealing with quantities like density and velocity, which at a given instant have specific values at every point in the fluid or other continuously distributed material. The density (rv ,t) is a scalar field in the sense that it has a scalar value at every point, while the velocity v (rv ,t) is a vector field, since it has a direction as well as a magnitude at every point. Fig. 1: A surface element at a point in a continuum. The surface stress is a more complicated type of quantity. The reason for this is that one cannot talk of the stress at a point without first defining the particular surface through v that point on which the stress acts. A small fluid surface element centered at the point r is defined by its area A (the prefix indicates an infinitesimal quantity) and by its outward v v unit normal vector n .