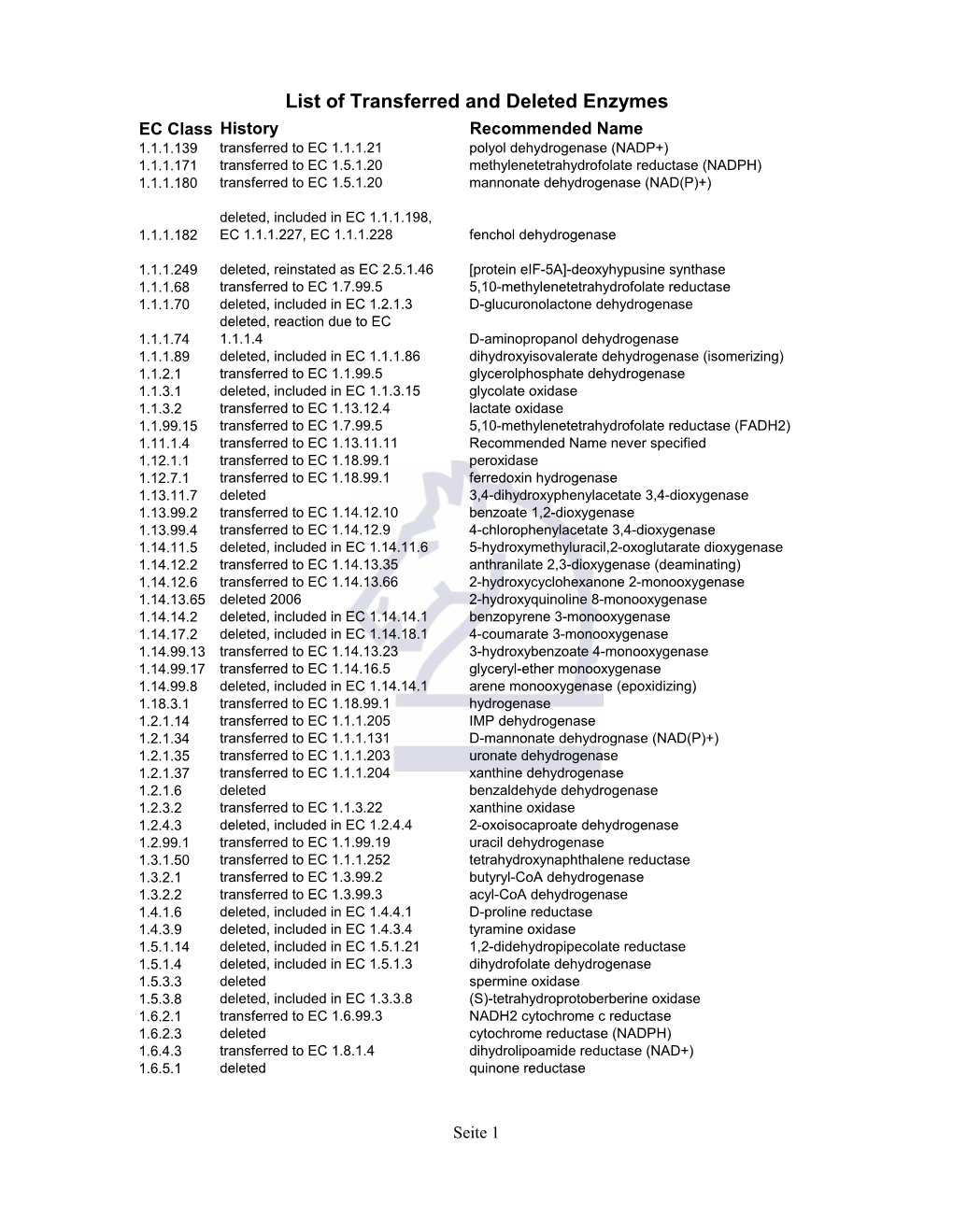
List of Transferred and Deleted Enzymes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THESIS Entitled Studies on Fraction 1 Protein of Beta Vulgaris Submitted for the Degree of DOCTOR of PHILOSOPHY by Kenneth Edwar
THESIS entitled Studies on Fraction 1 Protein of Beta vulgaris Submitted for the degree of DOCTOR OF PHILOSOPHY by Kenneth Edward Moon 1 970 TABLE OF CONTENTS Acknowledgements i Abbreviations i Summary ii Chapter 1 Introduction 1.1 Carbon Dioxide Fixation in the Calvin • 1 Cycle 1.2 Some Properties of RuDPcase and Similarity 2 to Fraction 1 Protein 1.3 Cellular Location of Fraction 1 Protein 3 1.4 The Mechanism of Action of RuDPcase 4 1.5 Structure of Fraction 1 Protein from 13 Higher Plants 1.6 Relationship of Structure to Enzymic 14 Activity and Role of Sulphydryl Groups 1.7 Studies on RuDPcase from Sources other 17 than the Higher Plants 1.8 Comparison of Some Kinetic and Physical 21 Constants of RuDPcase from Different Sources 1.9 Aim of This Research Project 23 Chapter 2 Methods and Materials 2.1 Plant Material 24 2.2 Preparation of Chloroplasts and Isolation 24 of Fraction 1 Protein 2.3 Concentrating Protein Solutions 26 2.4 Preparation of Reduced and Carboxy- 27 methylated Fraction 1 Protein 2.5 Preparation of Maleyl-carboxymethyl 28 Fraction 1 Protein 2.6 Removal of Maleyl Groups 29 2.7 Gel Electrophoresis 30 2.7 (i) Polyacrylamide Disk Electrophoresis 30 (ii) Polyacrylamide Electrophoresis in 31 the presence of SDS (iii) Slab Acrylamide Gel Electrophoresis 32 (iv) Isoelectric Focusing 33 2.8 Ribulose-1,5-diphosphate Carboxylase 3^ Assay 2.9 Preparation of the S-Sulphenylsulphanate 34 Derivative of Fraction 1 Protein 2.10 Edman Degradations 35 2.11 Hydrazinolysis 35 2.12 Isolation of Blocked N-Terminal Peptides 36 2.13 Tryptic -

Kinetics of Ornithine Carbamoyltransferase
15// 454 BIOCHEMICAL SOCIETY TRANSACTIONS Ammonia stimulation of malate dehydrogenase from Bacilllls slearolhermophillls: generation of an apparent aspartate dehydrogenase activity C. N. G. SCHMIDT* and L. JER VISt Attempts to purify aspartate dehydrogenase by salt fraction• • Departmel/t 0/ Botal/Y. Rothamsted Experimel/tal Statiol/. ation. chromatography on Cibacron Blue 3GA-Sepharose or \ Harpel/del/. Herts. A L5 2JQ. U.K .. al/d t Departmel/t 0/ Procion Red HE-3B-Scpharose. affinity chromatography on Biology. Paisley College o/Tee/mology. High Street. Paisley 5'-AMP-Sepharose and gel filtration through Sephacryl S-300 PA I 2BE. Re/l(rewshire. Seollal/d. U.K. always resulted in co-purification of malate dehydrogenase. ' Purification of malate dehydrogenase to near-Ifomogeneity by During investigations into the enzymology of ammonia assimi• the procedure of Wright & Sundaram (1979) yielded a product lation by Baeil/lls stearolhermophillls strain PH24 (Buswell & that was more than 90% pure but still appeared to contain Twomey. 1975). we detected high amounts of an apparent aspartate dehydrogenase in that the addition of ammonia to the aspartate dehydrogenase activity. The enzyme appeared to be malate dehydrogenase assay system caused a 2-fold increase in capable of catalysing the reductive ami nation of oxaloacetic acid the rate of NADH oxidation. Attempts to stain polyacrylamide with NA DH as cofactor. but could not catalyse the oxidative gels for aspartate dehydrogenase activity by using I.-aspartate. deamination of I.-aspartic acid. At low concentrations of NI~4C1 NAD+., Nitro Blue Tetrazolium and phenazonium metho• as the sole nitrogen source in the culture medium. the most sulphate were unsuccessful. -

Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae
Journal of Fungi Article Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae Mohammad Sayari 1,2,*, Magrieta A. van der Nest 1,3, Emma T. Steenkamp 1, Saleh Rahimlou 4 , Almuth Hammerbacher 1 and Brenda D. Wingfield 1 1 Department of Biochemistry, Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa; [email protected] (M.A.v.d.N.); [email protected] (E.T.S.); [email protected] (A.H.); brenda.wingfi[email protected] (B.D.W.) 2 Department of Plant Science, University of Manitoba, 222 Agriculture Building, Winnipeg, MB R3T 2N2, Canada 3 Biotechnology Platform, Agricultural Research Council (ARC), Onderstepoort Campus, Pretoria 0110, South Africa 4 Department of Mycology and Microbiology, University of Tartu, 14A Ravila, 50411 Tartu, Estonia; [email protected] * Correspondence: [email protected]; Fax: +1-204-474-7528 Abstract: Terpenes represent the biggest group of natural compounds on earth. This large class of organic hydrocarbons is distributed among all cellular organisms, including fungi. The different classes of terpenes produced by fungi are mono, sesqui, di- and triterpenes, although triterpene ergosterol is the main sterol identified in cell membranes of these organisms. The availability of genomic data from members in the Ceratocystidaceae enabled the detection and characterization of the genes encoding the enzymes in the mevalonate and ergosterol biosynthetic pathways. Using Citation: Sayari, M.; van der Nest, a bioinformatics approach, fungal orthologs of sterol biosynthesis genes in nine different species M.A.; Steenkamp, E.T.; Rahimlou, S.; of the Ceratocystidaceae were identified. -

Product Profiles of Egyptian Henbane Premnaspirodiene
The Journal of Antibiotics (2016) 69, 524–533 & 2016 Japan Antibiotics Research Association All rights reserved 0021-8820/16 www.nature.com/ja ORIGINAL ARTICLE Biosynthetic potential of sesquiterpene synthases: product profiles of Egyptian Henbane premnaspirodiene synthase and related mutants Hyun Jo Koo1,3, Christopher R Vickery1,2,3,YiXu1, Gordon V Louie1, Paul E O'Maille1, Marianne Bowman1, Charisse M Nartey1, Michael D Burkart2 and Joseph P Noel1 The plant terpene synthase (TPS) family is responsible for the biosynthesis of a variety of terpenoid natural products possessing diverse biological functions. TPSs catalyze the ionization and, most commonly, rearrangement and cyclization of prenyl diphosphate substrates, forming linear and cyclic hydrocarbons. Moreover, a single TPS often produces several minor products in addition to a dominant product. We characterized the catalytic profiles of Hyoscyamus muticus premnaspirodiene synthase (HPS) and compared it with the profile of a closely related TPS, Nicotiana tabacum 5-epi-aristolochene synthase (TEAS). The profiles of two previously studied HPS and TEAS mutants, each containing nine interconverting mutations, dubbed HPS-M9 and TEAS- M9, were also characterized. All four TPSs were compared under varying temperature and pH conditions. In addition, we solved the X-ray crystal structures of TEAS and a TEAS quadruple mutant complexed with substrate and products to gain insight into the enzymatic features modulating product formation. These informative structures, along with product profiles, -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

(12) United States Patent (10) Patent No.: US 9,115,366 B2 Tissier Et Al
USOO9115366B2 (12) United States Patent (10) Patent No.: US 9,115,366 B2 Tissier et al. (45) Date of Patent: *Aug. 25, 2015 (54) SYSTEM FOR PRODUCING TERPENOIDS IN WO WO99,38957 * 8, 1999 .......... C12N 15/82 PLANTS WO WO99,38957 A1 8/1999 WO WOOOf 17327 A3 3.2000 Fre WO WO 01/20008 A2 3, 2001 (75) Inventors: Alain Tissier, Pertuis (FR); Christophe WO WO 2004/111183 A2 12/2004 Sallaud, Montpellier (FR); Denis WO WO 2006/04.0479 4/2006 Rontein,ontein, GreouxG les Bains (FR(FR) OTHER PUBLICATIONS (73) Assignee: PHILIP MORRIS PRODUCTS S.A., Aharoni, Aetal. The Plant Cell (Dec. 2003), vol. 15: pp. 2866-2884.* Neuchatel (CH) Besumbes, O. et al. Biotechnology and Bioengineering; Oct. 20. 2004; vol. 88, No. 2: pp. 168-175.* (*) Notice: Subject to any disclaimer, the term of this Wang, E. et al. Nature Biotechnology, Apr. 2001; vol. 19, pp. 371 patent is extended or adjusted under 35 37.4% U.S.C. 154(b) by 900 days. Wang, E. et al. Journal of Experimental Botany, Sep. 2002, vol. 53, No. 376; pp. 1891-1897.* This patent is Subject to a terminal dis Walker K. et al. Phytochemistry (2001) vol. 58; pp. 1-7.* claimer. Gutiérrez-Alcalá et al., A versatile promoter for the expression of proteins in glandular and non-glandular trichomes from a variety of plants, 56 J of Exp Botany No. 419, 2487-2494 (2005).* (21) Appl. No.: 11/814,943 Besumbes et al. (Metabolic Engineering of Isoprenoid Biosynthesis in Arabidopsis for the Production of Taxadiene, the First Committed (22) PCT Filed: Jan. -

Catalase: Bioinformatics Analyses of One of the Key Enzymes in Hydrogen Peroxide Metabolism
6–71RYHPEHU 2019, Brno, Czech Republic Catalase: Bioinformatics analyses of one of the key enzymes in hydrogen peroxide metabolism Michaela Kameniarova, Romana Kopecka Department of Molecular Biology and Radiobiology Mendel University in Brno Zemedelska 1, 613 00 Brno CZECH REPUBLIC [email protected] Abstract: Catalases (CAT) are family of important antioxidant enzymes present in different isoforms and responsible for scavenging of hydrogen peroxide in almost all aerobically living organisms. In plants, they were found both in unicellular and multicellular species. Here, we performed bioinformatics analysis and analysed catalase evolutionary relationship and expression patterns. By comparing expression profiles of CATs and expression profiles of genes related to abiotic stimuli we found that almost 50% of light signalling genes were co-expressed with CATs. Further, by datamining in available resources and structural modelling we pinpointed candidate amino acid residues responsible for CAT thermostability. Key Words: catalase, H2O2, phylogenetics, abiotic stress, stability INTRODUCTION Plants contain several types of enzymes involved in H2O2 metabolism. These include catalases, ascorbate peroxidases, peroxiredoxins, glutathione/thioredoxin peroxidases, and glutathione S- transferases, but only catalases do not require additional cellular reductants (Mhamdi et al. 2010). Catalases are present almost in all aerobically respiring organisms. Within the cell environment, catalases are localized in all major sites of H2O2 production, mostly at peroxisomes, but they were detected in cytosol, mitochondria, and chloroplasts as well (Sharma and Ahmad 2014). Catalases (CAT, 1.11.1.6) are antioxidant enzymes that catalyse decomposition of hydrogen peroxide to water and molecular oxygen (2H2O2 → 2H2O + O2), an important process in defending cells against oxidative damage caused by reactive oxygen species (ROS; Alfonso-Prieto et al. -

Effect of Short Time Exposure of Rats to Extreme Low Temperature on Some Plasma and Liver Enzymes
Bull Vet Inst Pulawy 50, 121-124, 2006 EFFECT OF SHORT TIME EXPOSURE OF RATS TO EXTREME LOW TEMPERATURE ON SOME PLASMA AND LIVER ENZYMES EWA ROMUK1, EWA BIRKNER1, BRONISŁAWA SKRZEP-POLOCZEK1, LESZEK JAGODZIŃSKI2, AGATA STANEK2, BERNADETA WIŚNIOWSKA3 AND ALEKSANDER SIEROŃ2 1Department of Biochemistry in Zabrze, 2Clinic of Internal Diseases, Angiology and Physical Medicine in Bytom, Medical University of Silesia, 40-006 Katowice, Poland 3Center of Cryotherapy, 41-709 Ruda Śląska, Poland e-mail: [email protected] Received for publication October 10, 2005. Abstract protocol of the studies was reviewed and approved by the Bioethical Committee of Medical University of The aim of the study was to search the influence of Silesia in Katowice. Animals were supplied by the cryotherapy on liver enzyme activity in experimental rat Experimental Animal Farm. The rats underwent two model. The first group of rats was exposed 1 min daily to - week environmental adaptation cycle. After this period 90°C for 5 d, the second group was exposed 1 min daily to - the animals were randomized into 3 equal groups. Group 90°C for 10 d and the control group was not exposed to low I - rats exposed to -90°C for 5 d; group II - rats exposed temperature. A statistically significant increase in the activity to -90°C for 10 d and group III - control rats – without of glutamate dehydrogenase, sorbitol dehydrogenase, malate exposure to cryotherapy. All the groups were fed dehydrogenase, ornithine transcarbamoylase and arginase was observed in the plasma and liver. The obtained results indicate standard rat chow ad libitum. the influence of low temperature on liver metabolism. -

B Number Gene Name Mrna Intensity Mrna
sample) total list predicted B number Gene name assignment mRNA present mRNA intensity Gene description Protein detected - Membrane protein membrane sample detected (total list) Proteins detected - Functional category # of tryptic peptides # of tryptic peptides # of tryptic peptides detected (membrane b0002 thrA 13624 P 39 P 18 P(m) 2 aspartokinase I, homoserine dehydrogenase I Metabolism of small molecules b0003 thrB 6781 P 9 P 3 0 homoserine kinase Metabolism of small molecules b0004 thrC 15039 P 18 P 10 0 threonine synthase Metabolism of small molecules b0008 talB 20561 P 20 P 13 0 transaldolase B Metabolism of small molecules chaperone Hsp70; DNA biosynthesis; autoregulated heat shock b0014 dnaK 13283 P 32 P 23 0 proteins Cell processes b0015 dnaJ 4492 P 13 P 4 P(m) 1 chaperone with DnaK; heat shock protein Cell processes b0029 lytB 1331 P 16 P 2 0 control of stringent response; involved in penicillin tolerance Global functions b0032 carA 9312 P 14 P 8 0 carbamoyl-phosphate synthetase, glutamine (small) subunit Metabolism of small molecules b0033 carB 7656 P 48 P 17 0 carbamoyl-phosphate synthase large subunit Metabolism of small molecules b0048 folA 1588 P 7 P 1 0 dihydrofolate reductase type I; trimethoprim resistance Metabolism of small molecules peptidyl-prolyl cis-trans isomerase (PPIase), involved in maturation of b0053 surA 3825 P 19 P 4 P(m) 1 GenProt outer membrane proteins (1st module) Cell processes b0054 imp 2737 P 42 P 5 P(m) 5 GenProt organic solvent tolerance Cell processes b0071 leuD 4770 P 10 P 9 0 isopropylmalate -

Biochemistry I Enzymes
BIOCHEMISTRY I 3rd. Stage Lec. ENZYMES Biomedical Importance: Enzymes, which catalyze the biochemical reactions, are essential for life. They participate in the breakdown of nutrients to supply energy and chemical building blocks; the assembly of those building blocks into proteins, DNA, membranes, cells, and tissues; and the harnessing of energy to power cell motility, neural function, and muscle contraction. The vast majority of enzymes are proteins. Notable exceptions include ribosomal RNAs and a handful of RNA molecules imbued with endonuclease or nucleotide ligase activity known collectively as ribozymes. The ability to detect and to quantify the activity of specific enzymes in blood, other tissue fluids, or cell extracts provides information that complements the physician’s ability to diagnose and predict the prognosis of many diseases. Further medical applications include changes in the quantity or in the catalytic activity of key enzymes that can result from genetic defects, nutritional deficits, tissue damage, toxins, or infection by viral or bacterial pathogens (eg, Vibrio cholerae). Medical scientists address imbalances in enzyme activity by using pharmacologic agents to inhibit specific enzymes and are investigating gene therapy as a means to remedy deficits in enzyme level or function. In addition to serving as the catalysts for all metabolic processes, their impressive catalytic activity, substrate specificity, and stereospecificity enable enzymes to fulfill key roles in additional processes related to human health and well-being. Proteases and amylases augment the capacity of detergents to remove dirt and stains, and enzymes play important roles in producing or enhancing the nutrient value of food products for both humans and animals. -

(12) Patent Application Publication (10) Pub. No.: US 2003/0082511 A1 Brown Et Al
US 20030082511A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0082511 A1 Brown et al. (43) Pub. Date: May 1, 2003 (54) IDENTIFICATION OF MODULATORY Publication Classification MOLECULES USING INDUCIBLE PROMOTERS (51) Int. Cl." ............................... C12O 1/00; C12O 1/68 (52) U.S. Cl. ..................................................... 435/4; 435/6 (76) Inventors: Steven J. Brown, San Diego, CA (US); Damien J. Dunnington, San Diego, CA (US); Imran Clark, San Diego, CA (57) ABSTRACT (US) Correspondence Address: Methods for identifying an ion channel modulator, a target David B. Waller & Associates membrane receptor modulator molecule, and other modula 5677 Oberlin Drive tory molecules are disclosed, as well as cells and vectors for Suit 214 use in those methods. A polynucleotide encoding target is San Diego, CA 92121 (US) provided in a cell under control of an inducible promoter, and candidate modulatory molecules are contacted with the (21) Appl. No.: 09/965,201 cell after induction of the promoter to ascertain whether a change in a measurable physiological parameter occurs as a (22) Filed: Sep. 25, 2001 result of the candidate modulatory molecule. Patent Application Publication May 1, 2003 Sheet 1 of 8 US 2003/0082511 A1 KCNC1 cDNA F.G. 1 Patent Application Publication May 1, 2003 Sheet 2 of 8 US 2003/0082511 A1 49 - -9 G C EH H EH N t R M h so as se W M M MP N FIG.2 Patent Application Publication May 1, 2003 Sheet 3 of 8 US 2003/0082511 A1 FG. 3 Patent Application Publication May 1, 2003 Sheet 4 of 8 US 2003/0082511 A1 KCNC1 ITREXCHO KC 150 mM KC 2000000 so 100 mM induced Uninduced Steady state O 100 200 300 400 500 600 700 Time (seconds) FIG. -

In TCR-Stimulated Thymocytes Induction of Chromosomal DNA Degradation Caspase-Activated Deoxyribonuclease in the Possible Involv
Possible Involvement of Cyclophilin B and Caspase-Activated Deoxyribonuclease in the Induction of Chromosomal DNA Degradation in TCR-Stimulated Thymocytes This information is current as of September 28, 2021. Takuya Nagata, Hiroyuki Kishi, Qing Li Liu, Tomoyasu Yoshino, Tadashi Matsuda, Zhe Xiong Jin, Kimie Murayama, Kazuhiro Tsukada and Atsushi Muraguchi J Immunol 2000; 165:4281-4289; ; doi: 10.4049/jimmunol.165.8.4281 Downloaded from http://www.jimmunol.org/content/165/8/4281 References This article cites 42 articles, 23 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/165/8/4281.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Possible Involvement of Cyclophilin B and Caspase-Activated Deoxyribonuclease in the Induction of Chromosomal DNA Degradation in TCR-Stimulated Thymocytes1 Takuya Nagata,* Hiroyuki Kishi,* Qing Li Liu,* Tomoyasu Yoshino,* Tadashi Matsuda,* Zhe Xiong Jin,* Kimie Murayama,‡ Kazuhiro Tsukada,† and Atsushi Muraguchi2* TCR engagement of immature CD4؉CD8؉ thymocytes induces clonal maturation (positive selection) as well as clonal deletion (negative selection) in the thymus.