Differences and Similarities Between Disseminated Intravascular
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Thrombotic Thrombocytopenic Purpura and Systemic Lupus Erythematosus: Successful Management of a Rare Presentation
Indian J Crit Care Med July-September 2008 Vol 12 Issue 3 Case Report Thrombotic thrombocytopenic purpura and systemic lupus erythematosus: Successful management of a rare presentation Pratish George, Jasmine Das, Basant Pawar1, Naveen Kakkar2 Thrombotic thrombocytopenic purpura (TTP) and systemic lupus erythematosus (SLE) very rarely present simultaneously and pose a diagnostic and therapeutic dilemma to the critical care team. Prompt diagnosis and management with plasma exchange and immunosuppression is life-saving. A patient critically ill with TTP and SLE, successfully managed in the acute period of illness with plasma exchange, steroids and Abstract mycophenolate mofetil is described. Key words: Plasma exchange, systemic lupus erythematosus, thrombotic thrombocytopenic purpura Introduction Case Report Systemic lupus erythematosis (SLE) is diagnosed by A 30-year-old lady was admitted with fever and the presence of four or more of the following criteria, jaundice. A week earlier she had undergone an serially or simultaneously: malar rash, discoid rash, uncomplicated medical termination of pregnancy at photosensitivity, oral ulcers, non erosive arthritis, serositis, another hospital, at 13 weeks of gestation. She had an renal abnormalities including proteinuria or active urinary uneventful pregnancy with twins two years earlier and sediments, neuropsychiatric features, hematological the twins were diagnosed to have thalassemia major. abnormalities including hemolytic anemia, leucopenia, She was subsequently diagnosed to have thalassemia lymphopenia and thrombocytopenia, immunological minor and her husband had thalassemia minor trait. No markers like anti-ds DNA or anti-Smith antibody and high earlier history of spontaneous Þ rst trimester abortions Antinuclear antibody titres. Thrombotic thrombocytopenic was present. purpura (TTP) in patients with SLE is extremely rare. -

A Cross Sectional Study of Cutaneous Manifestations in 300 Patients of Diabetes Mellitus
International Journal of Advances in Medicine Khuraiya S et al. Int J Adv Med. 2019 Feb;6(1):150-154 http://www.ijmedicine.com pISSN 2349-3925 | eISSN 2349-3933 DOI: http://dx.doi.org/10.18203/2349-3933.ijam20190122 Original Research Article A cross sectional study of cutaneous manifestations in 300 patients of diabetes mellitus Sandeep Khuraiya1*, Nancy Lal2, Naseerudin3, Vinod Jain3, Dilip Kachhawa3 1Department of Dermatology, 2Department of Radiation Oncology , Gandhi Medical College, Bhopal, Madhya Pradesh, India 3Department of Dermatology, Dr. SNMC, Jodhpur, Rajasthan, India Received: 13 December 2018 Accepted: 05 January 2019 *Correspondence: Dr. Sandeep Khuraiya, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Diabetes Mellitus (DM) is a worldwide problem and one of the most common endocrine disorder. The skin is affected by both the acute metabolic derangements and the chronic degenerative complications of diabetes. Methods: The present study was a one-year cross sectional study from January 2014 to December 2014. All confirmed cases of DM with cutaneous manifestations irrespective of age, sex, duration of illness and associated diseases, willing to participate in the study were included in the study. Routine haematological and urine investigations, FBS, RBS and HbA1c levels were carried out in all patients. Results: A total of 300 patients of diabetes mellitus with cutaneous manifestations were studied. -

Note for Guidance on Clinical Investigation of Medicinal Products for the Treatment of Peripheral Arterial Occlusive Disease
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 25 April 2002 CPMP/EWP/714/98 rev 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT OF PERIPHERAL ARTERIAL OCCLUSIVE DISEASE DISCUSSION IN THE EFFICACY WORKING PARTY September 1999 – September 2000 TRANSMISSION TO CPMP November 2000 RELEASE FOR CONSULTATION November 2000 DEADLINE FOR COMMENTS May 2001 DISCUSSION IN THE EFFICACY WORKING PARTY November 2001 – February 2002 TRANSMISSION TO CPMP April 2002 ADOPTION BY CPMP April 2002 DATE FOR COMING INTO OPERATION October 2002 Note: This revised Note for guidance will replace the previous Note for guidance (CPMP/EWP/233/95) , adopted in November 1995. 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 8613 E-mail: [email protected] http://www.emea.eu.int EMEA 2002 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged NOTE FOR GUIDANCE ON CLINICAL INVESTIGATIONS OF MEDICINAL PRODUCTS IN THE TREATMENT OF CHRONIC PERIPHERAL ARTERIAL OCCLUSIVE DISEASE TABLE OF CONTENTS 1. INTRODUCTION .................................................................................................... 4 2. GENERAL CONSIDERATIONS REGARDING PAOD TRIALS..................... 4 2.1 PAOD Classification and Epidemiological Background ...................................... 4 2.2 Clinical Trial Features ............................................................................................ -
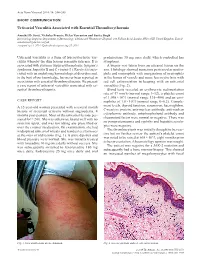
Urticarial Vasculitis Associated with Essential Thrombocythaemia
Acta Derm Venereol 2014; 94: 244–245 SHORT COMMUNICATION Urticarial Vasculitis Associated with Essential Thrombocythaemia Annabel D. Scott, Nicholas Francis, Helen Yarranton and Sarita Singh Dermatology Registrar, Department of Dermatology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom. E-mail: [email protected] Accepted Apr 3, 2013; Epub ahead of print Aug 27, 2013 Urticarial vasculitis is a form of leucocytoclastic vas- prednisolone 30 mg once daily, which controlled her culitis whereby the skin lesions resemble urticaria. It is symptoms. associated with systemic lupus erythematosus, Sjögren’s A biopsy was taken from an uticarial lesion on the syndrome, hepatitis B and C viruses (1). Rarely it is asso- arm. Histology showed numerous perivascular neutro- ciated with an underlying haematological disorders and, phils and eosinophils with margination of neutrophils to the best of our knowledge, has never been reported in in the lumen of vessels and some leucocytoclasis with association with essential thrombocythaemia. We present red cell extravasation in keeping with an urticarial a case report of urticarial vasculitis associated with es- vasculitis (Fig. 2). sential thrombocythaemia. Blood tests revealed an erythrocyte sedimentation rate of 47 mm/h (normal range 1–12), a platelet count of 1,098 × 109/l (normal range 135–400) and an eosi- CASE REPORT nophilia of 1.0 × 109/l (normal range 0–0.2). Comple- A 32-year-old woman presented with a several month ment levels, thyroid function, serum iron, haemoglobin, history of recurrent urticaria without angioedema, 4 C-reactive protein, anti-nuclear antibody, anti-nuclear months post-partum. -

Immune-Pathophysiology and -Therapy of Childhood Purpura
Egypt J Pediatr Allergy Immunol 2009;7(1):3-13. Review article Immune-pathophysiology and -therapy of childhood purpura Safinaz A Elhabashy Professor of Pediatrics, Ain Shams University, Cairo Childhood purpura - Overview vasculitic disorders present with palpable Purpura (from the Latin, purpura, meaning purpura2. Purpura may be secondary to "purple") is the appearance of red or purple thrombocytopenia, platelet dysfunction, discolorations on the skin that do not blanch on coagulation factor deficiency or vascular defect as applying pressure. They are caused by bleeding shown in table 1. underneath the skin. Purpura measure 0.3-1cm, A thorough history (Table 2) and a careful while petechiae measure less than 3mm and physical examination (Table 3) are critical first ecchymoses greater than 1cm1. The integrity of steps in the evaluation of children with purpura3. the vascular system depends on three interacting When the history and physical examination elements: platelets, plasma coagulation factors suggest the presence of a bleeding disorder, and blood vessels. All three elements are required laboratory screening studies may include a for proper hemostasis, but the pattern of bleeding complete blood count, peripheral blood smear, depends to some extent on the specific defect. In prothrombin time (PT) and activated partial general, platelet disorders manifest petechiae, thromboplastin time (aPTT). With few exceptions, mucosal bleeding (wet purpura) or, rarely, central these studies should identify most hemostatic nervous system bleeding; -

IWGDF Guideline on Diagnosis, Prognosis and Management of Peripheral Artery Disease in Patients with a Foot Ulcer and Diabetes
IWGDF Guideline on diagnosis, prognosis and management of peripheral artery disease in patients with a foot ulcer and diabetes Part of the 2019 IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease IWGDF Guidelines AUTHORS Robert J. Hinchlife1, Rachael O. Forsythe2, Jan Apelqvist3, Ed J. Boyko4, Robert Fitridge5, Joon Pio Hong6, Konstantinos Katsanos7, Joseph L. Mills8, Sigrid Nikol9, Jim Reekers10, Maarit Venermo11, R. Eugene Zierler12, Nicolaas C. Schaper13 on behalf of the International Working Group on the Diabetic Foot (IWGDF) INSTITUTIONS 1 Bristol Centre for Surgical Research, University of Bristol, Bristol, UK 2 British Heart Foundation / University of Edinburgh Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, Scotland, UK 3 Department of Endocrinology, University Hospital of Malmö, Sweden 4 Seattle Epidemiologic Research and Information Centre-Department of Veterans Afairs Puget Sound Health Care System and the University of Washington, Seattle, Washington, USA 5 Vascular Surgery, The University of Adelaide, Adelaide, South Australia, Australia 6 Asan Medical Center University of Ulsan, Seoul, Korea 7 Patras University Hospital School of Medicine, Rion, Patras, Greece 8 SALSA (Southern Arizona Limb Salvage Alliance), University of Arizona Health Sciences Center, Tucson, Arizona, USA 9 Asklepios Klinik St. Georg, Hamburg, Germany 10 Department of Vascular Radiology, Amsterdam Medical Centre, The Netherlands 11 Helsinki University Hospital, University of Helsinki, Finland 12 Department of Surgery, University of Washington, Seattle, Washington, USA 13 Div. Endocrinology, MUMC+, CARIM and CAPHRI Institute, Maastricht, The Netherlands KEYWORDS diabetic foot; foot ulcer; guidelines; peripheral artery disease; surgery; diagnosis; prognosis; vascular disease www.iwgdfguidelines.org IWGDF PAD Guideline ABSTRACT The International Working Group on the Diabetic Foot (IWGDF) has published evidence-based guidelines on the prevention and management of diabetic foot disease since 1999. -

Coronary Microvascular Dysfunction
Journal of Clinical Medicine Review Coronary Microvascular Dysfunction Federico Vancheri 1,*, Giovanni Longo 2, Sergio Vancheri 3 and Michael Henein 4,5,6 1 Department of Internal Medicine, S.Elia Hospital, 93100 Caltanissetta, Italy 2 Cardiovascular and Interventional Department, S.Elia Hospital, 93100 Caltanissetta, Italy; [email protected] 3 Radiology Department, I.R.C.C.S. Policlinico San Matteo, 27100 Pavia, Italy; [email protected] 4 Institute of Public Health and Clinical Medicine, Umea University, SE-90187 Umea, Sweden; [email protected] 5 Department of Fluid Mechanics, Brunel University, Middlesex, London UB8 3PH, UK 6 Molecular and Nuclear Research Institute, St George’s University, London SW17 0RE, UK * Correspondence: [email protected] Received: 10 August 2020; Accepted: 2 September 2020; Published: 6 September 2020 Abstract: Many patients with chest pain undergoing coronary angiography do not show significant obstructive coronary lesions. A substantial proportion of these patients have abnormalities in the function and structure of coronary microcirculation due to endothelial and smooth muscle cell dysfunction. The coronary microcirculation has a fundamental role in the regulation of coronary blood flow in response to cardiac oxygen requirements. Impairment of this mechanism, defined as coronary microvascular dysfunction (CMD), carries an increased risk of adverse cardiovascular clinical outcomes. Coronary endothelial dysfunction accounts for approximately two-thirds of clinical conditions presenting with symptoms and signs of myocardial ischemia without obstructive coronary disease, termed “ischemia with non-obstructive coronary artery disease” (INOCA) and for a small proportion of “myocardial infarction with non-obstructive coronary artery disease” (MINOCA). More frequently, the clinical presentation of INOCA is microvascular angina due to CMD, while some patients present vasospastic angina due to epicardial spasm, and mixed epicardial and microvascular forms. -

Appendix Search Strategy Treatment of Hemophilia.Pdf
Appendix Search strategies Hemophilia – general aspects PubMed (NLM) September 2009 Von Willebrand disease (TiAb) AND Controlled clinical trial (PT) NOT Purpura, Thrombocytopenic (Me) Angiohemophilia (TiAb) Meta analysis (PT) Blood coagulation disorders (Me) Randomized controlled trial (PT) Hemophilia (TiAb) Systematic (SB) Haemophilia (TiAb) Bleeding disorder (TiAb) Random* (Ti) Bleeding disorders (TiAb) OR Control* (Ti) NOT Medline (SB) ("controlled clinical trial"[Publication Type] OR "meta analysis"[Publication Type] OR "randomized controlled trial"[Publication Type] OR systematic[sb] OR ((random*[Title] OR control*[Title]) NOT Medline[sb])) AND ("von Willebrand Disease"[title/abstract] OR "angiohemophilia"[title/Abstract] OR "Blood Coagulation Disorders"[Mesh terms] OR "hemophilia"[title/abstract] OR "haemophilia"[title/abstract] OR "bleeding disorder"[title/abstract] OR "bleeding disorders"[Title/abstract]) NOT "Purpura, Thrombocytopenic"[MeSH Terms] 211 Hemophilia – general aspects Embase.com (Elsevier) September 2009 Blood clotting factor deficiency (Exp,MJR) AND Clinical trial (Exp) NOT Thrombocytopenic purpura (Exp) Von Willebrand disease (Ti) Intervention study (De) Angiohemophilia (Ti) Longitudinal study (De) Angiohaemophilia (Ti) Prospective study (De) Hemophilia (Ti) Meta analysis (De) Haemophilia (Ti) Systematic review (De) Bleeding disorder (Ti) Random* (Ti) Bleeding disorders (Ti) Control* (Ti) ('blood clotting factor deficiency'/exp/mjOR 'von willebrand disease':ti OR 'angiohemophilia':ti OR 'angiohaemophilia':ti OR 'hemophilia':ti -

Immune Thrombocytopenic Purpura with Subsequent Development of JAK2 V617F-Positive Essential Thrombocythemia: Case Report
ISSN: 2640-7914 DOI: https://dx.doi.org/10.17352/ahcrr CLINICAL GROUP Received: 13 July, 2021 Case Report Accepted: 03 August, 2021 Published: 04 August, 2021 *Corresponding author: Marisabel Hurtado-Castillo, Immune thrombocytopenic PGY-3, Internal Medicine, Department of Medicine at NYU, 536 Ovington Avenue Apartment 3, Brooklyn NYC 11209, USA, Tel: 718-312-9641; purpura with subsequent Email: Keywords: Immune thrombocytopenic purpura; development of JAK2 Essential thrombocythemia; JAK-STAT signaling path- way; JAK2(V617F) mutation V617F-positive essential https://www.peertechzpublications.com thrombocythemia: Case Report Marisabel Hurtado-Castillo1*, Brian Flaherty2 and Morris Jrada2 1PGY-3, Internal Medicine, Department of Medicine at NYU, Brooklyn, NY, USA 2Clinical Assistant Professor, Department of Medicine at NYU Grossman School of Medicine, Brooklyn, NY, USA Abstract The sequential occurrence of Immune Thrombocytopenic Purpura (ITP) and Essential Thrombocythemia (ET) has been reported in the literature on a few occasions, as these are two hematologic disorders with distinct etiologies and patients usually have contrasting clinical presentations. Our case highlights the sequential occurrence of ITP, followed by Janus kinase 2 (JAK2) (V617F)-positive ET in a 64-year-old white woman, after four years of follow-up. The pathophysiology relating to these two conditions is incompletely understood, however, JAK2(V617F) mutation has been found in all the cases reported. Early identifi cation of JAK2(V617F) mutation in a patient with a -

Thrombocytopenia.Pdf
THROMBOCYTOPENIA DIFFERENTIAL DIAGNOSIS FALSELY LOW PLATELET COUNT In vitro platelet clumping caused by EDTA-dependent agglutinins Giant platelets COMMON CAUSES OF THROMBOCYTOPENIA Pregnancy (gestational thrombocytopenia, preeclampsia) Drug-induced thrombocytopenia (i.e., heparin, quinidine, quinine, and sulfonamides) Viral infections (ie. HIV, rubella, infectious mononucleosis) Hypersplenism due to chronic liver disease Dilutional (massive transfusion) OTHER CAUSES OF THROMBOCYTOPENIA Myelodysplasia Congenital thrombocytopenia Thrombotic thrombocytopenic purpura (TTP) -hemolytic-uremic syndrome (HUS) Chronic disseminated intravascular coagulation (DIC) Autoimmune diseases, such as systemic lupus erythematosus-associated lymphoproliferative disorders (CLL and NHL) Sepsis Idiopathic thrombocytopenic purpura (ITP)* DIFFERENTIAL FOR THROMBOCYTOPENIA BASED ON CLINICAL SETTING CLINICAL SETTING DIFFERENTIAL DIAGNOSES Cardiac surgery Cardiopulmonary bypass, HIT, dilutional thrombocytopenia, PTP Interventional cardiac Abciximab or other IIb/IIIa blockers, HIT procedure Sepsis syndrome DIC, ehrlichiosis, sepsis, hemophagocytosis syndrome, drug-induced, misdiagnosed TTP, mechanical ventilation, pulmonary artery catheters Pulmonary failure DIC, hantavirus pulmonary syndrome, mechanical ventilation, pulmonary artery catheters Mental status TTP, ehrlichiosis changes/seizures Renal failure TTP, Dengue, HIT, DIC, HUS Continuous hemofiltration HIT, consumption by filter and tubing Cardiac failure HIT, drug-induced, pulmonary artery catheter Post-surgery -

Pathobiology of Thrombocytopenia and Bleeding in Patients with Wiskott-Aldrich Syndrome
TITLE: Pathobiology of Thrombocytopenia and Bleeding in Patients with Wiskott-Aldrich Syndrome Principal Investigator: James B. Bussel, MD IRB Protocol Number: 0801009600 ClinicalTrials.gov ID: NCT00909363 Compound Number: SB-497115 Development Phase: Phase II Effective Date: August 26th 2010 Updated: September 18, 2015 Protocol Versions: August 15, 2013 October 28, 2014 December 31, 2014 July 7, 2015 1 TABLE OF CONTENTS List of Abbreviations 4 1 INTRODUCTION 5 1.1 Background 5 1.2 Rationale 5 2 OBJECTIVE(S) 6 2.1 Primary Objective 6 2.2 Secondary Objectives 6 3 INVESTIGATIONAL PLAN 6 3.1 Study Design 6 3.2 Laboratory Testing 7 4 SUBJECT SELECTION AND WITHDRAWAL CRITERIA 8 4.1 Number of Subjects 8 4.2 Inclusion Criteria 8 4.3 Exclusion Criteria 8 4.4 Withdrawal Criteria 9 4.4.1 Study Stopping Rules 10 4.4.2 Patient Stopping Rules 10 5 STUDY TREATMENTS 10 5.1 Treatment Assignment 10 5.2 Product Accountability 11 5.3 Treatment Compliance 11 5.4 Concomitant Medications and Non-Drug Therapies 11 5.4.1 Permitted Medications and Non-Drug Therapies 11 5.4.2 Prohibited Medications and Non-Drug Therapies 11 5.5 Treatment after the End of the Study 11 5.6 Treatment of Investigational Product Overdose 11 5.7 Treatment Plan 12 6 STUDY ASSESSMENTS AND PROCEDURES 13 6.1 Critical Baseline Assessments 13 6.2 Efficacy 13 6.3 Safety 13 6.3.1 Liver chemistry stopping and follow-up criteria 15 6.4 Adverse Events 16 6.4.1 Definition of an AE 16 6.4.2 Definition of a SAE 17 6.4.3 Disease-Related Events and/or Disease-Related Outcomes Not Qualifying as SAEs -

Origin of the Microangiopathic Changes in Diabetes
ORIGIN OF THE MICROANGIOPATHIC CHANGES IN DIABETES A. H. BARNETT Birmingham SUMMARY its glycosidally linked disaccharide units. Such alterations The mechanism of development of microangiopathy is lead to abnormal packing of the peptide chains producing incompletely understood, but relates to a number of excessive leakiness of the membrane. The exact mech ultrastructural, biochemical and haemostatic processes. anisms of thickening and leakiness of basement mem These include capillary basement membrane thickening, brane and their relevance to diabetic complications are not non-enzymatic glycosylation, possibly increased free rad entirely clear, but appear to involve several biochemical ical activity, increased flux through the polyol pathway mechanisms. and haemostatic abnormalities. The central feature Non-enzymatic Glycosylation appears to be hyperglycaemia, which is causally related to the above processes and culminates in tissue ischaemia. In the presence of persistent hyperglycaemia glucose This article will briefly describe these processes and will chemically attaches to proteins non-enzymatically to form discuss possible pathogenic interactions which may lead a stable product (keto amine or Amadori product) of which to the development of the pathological lesion. glycosylated haemoglobin is the best-known example. In long-lived tissue proteins such as collagen the ketoamine Microangiopathy is a specific disorder of the small blood then undergoes a series of reactions resulting in the vessels which causes much morbidity and mortality in dia development of advanced glycosylation end products betic patients. Diabetic retinopathy is the commonest (AGE) (Fig. 1).5 AGE are resistant to degradation and con cause of blindness in the working population of the United tinue to accumulate indefinitely on long-lived proteins.