Native Or Introduced? Fossil Pollen and Spores May Say. an Example from the Azores Islands
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -
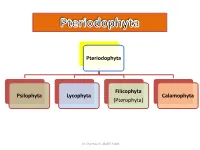
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Phyton Annales Rei Botanicae
©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at PHYTON ANNALES REI BOTANICAE VOL. 45, FASC. 1 PAG. 1--144 30. 6. 2005 Phyton (Horn, Austria) Vol. 45 Fasc. 1 1-8 30. 6. 2005 The Gametophyte-Sporophyte Junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta) By Hartmut H. HILGER*), Nancy KAPUSKAR**) and Wolfgang FREY*) With 6 Figures Received August 20, 2004 Key words: Lycopodiophyta, Pteridophyta, Selaginella, Selaginellales. - Anatomy;omy,, gametophyte-sporophytgame e junction, placental space. - Electron microscopy. Summary HILGER H. H., KAPUSKAR N. & FREY W. 2005. The gametophyte-sporophyte junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta). - Phyton (Horn, Austria) 45 (1): 1-8, with 6 figures. - English with German summary. The gametophyte-sporophyte junction in Selaginella martensii (Selaginellales, Lycopodiophyta) consists of a sporophytic conical foot embedded in the maternal gametophytic tissue. Both generations are separated by a narrow placental space *) Prof. Dr. H. H. HILGER, Prof. Dr. W. FREY, Institut für Biologie - Systematische Botanik und Pflanzengeographie - der Freien Universität Berlin, Altensteinstrae 6, D-14195 Berlin, Germany; e-mail: [email protected], [email protected] berlin.de **) Dr. N. KAPUSKAR, Institut für Spezielle Botanik und Botanischer Garten der Johannes Gutenberg-Universität, Bentzelweg 2, D-55099 Mainz; e-mail: [email protected] ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at filled with thin-walled collapsed cells of gametophytic origin. Gametophytic and sporophytic placental cells lack wall ingrowths, respectively transfer cells. Neither interdigitation nor intermingling of placental cells nor nacreous thickenings are de- veloped. The structure of the gametophyte-sporophyte junction in Selaginella mar- tensii resembles that of Isoetes boliviensis, the second lycopodiopside investigated, and thus differs from the junction described for pteridopsides and lycopods. -

Selaginella Wangpeishanii (Selaginellaceae), a New Lycophyte from a Limestone Cave in Guizhou, China
Phytotaxa 164 (3): 195–199 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2014 Magnolia Press ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.164.3.5 Selaginella wangpeishanii (Selaginellaceae), a new lycophyte from a limestone cave in Guizhou, China LI-BING ZHANG1 , QING-WEN SUN2 & HAI HE3* 1Chengdu Institute of Biology, Chinese Academy of Sciences, P.O. Box 416, Chengdu, Sichuan 610041, P. R. China and Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. 2Department of Pharmacy, Guiyang College of Traditional Chinese Medicine, Guiyang, Guizhou 550002, P. R. China 3College of Life Sciences, Chongqing Normal University, Shapingba, Chongqing 400047, P. R. China *Author for correspondence: e-mail: [email protected] Abstract Selaginella wangpeishanii, a new species of the lycophyte genus Selaginella (Selaginellaceae) from a limestone cave in southern Guizhou, China is described and illustrated. The new species is different from any species in the genus known so far in China by having some ultimate branches bearing microphylls in ascending order of trophophylls, sporophylls or sporophyll-like microphylls, and trophophylls (TST arrangement of microphylls). Morphologically, S. wangpeishanii is most similar to S. gebaueriana, but is distinct by smaller plants and serrulate margins of lateral trophophylls. Selaginella wangpeishanii is currently known only from a single population and is considered to be Critically Endangered (CR), based on IUCN Red List criteria. Key words: Selaginella wangpeishanii, Guizhou, TST arrangement of microphylls, IUCN Red List Introduction Selaginella Beauvois (1804: 478) (Selaginellaceae) is the largest lycophyte genus with about 750 species mainly distributed in tropical and subtropical areas (Jermy 1990). -

A New Species in the Tree Genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew Marshall, Thomas L.P
A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew Marshall, Thomas L.P. Couvreur, Abigail Summers, Nicolas Deere, W.R. Quentin Luke, Henry Ndangalasi, Sue Sparrow, David Johnson To cite this version: Andrew Marshall, Thomas L.P. Couvreur, Abigail Summers, Nicolas Deere, W.R. Quentin Luke, et al.. A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania. PhytoKeys, Pensoft, 2016, 63, pp.63-76. 10.3897/phytokeys.63.6262. hal-03275053 HAL Id: hal-03275053 https://hal.archives-ouvertes.fr/hal-03275053 Submitted on 30 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License A peer-reviewed open-access journal PhytoKeys 63: 63–76 (2016)A new species in the tree genus Polyceratocarpus (Annonaceae)... 63 doi: 10.3897/phytokeys.63.6262 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania Andrew R. Marshall1,2, Th omas L.P. Couvreur4,5,6, Abigail L. Summers1,2,3, Nicolas J. -

Identification of WOX Family Genes in Selaginella Kraussiana for Studies
ORIGINAL RESEARCH published: 05 February 2016 doi: 10.3389/fpls.2016.00093 Identification of WOX Family Genes in Selaginella kraussiana for Studies on Stem Cells and Regeneration in Lycophytes Yachao Ge1†,JieLiu1†, Minhuan Zeng1†,JianfengHe1,PengQin2, Hai Huang1 and Lin Xu1* 1 National Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences, Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China, 2 Department of Instrumentation Science and Engineering, Shanghai Jiao Tong University, Shanghai, China Plant stem cells give rise to all tissues and organs and also serve as the source for plant regeneration. The organization of plant stem cells has undergone a progressive change from simple to complex during the evolution of vascular plants. Most studies on plant stem cells have focused on model angiosperms, the most recently diverged branch of vascular plants. However, our knowledge of stem cell function in other vascular plants Edited by: John Love, is limited. Lycophytes and euphyllophytes (ferns, gymnosperms, and angiosperms) are University of Exeter, UK two existing branches of vascular plants that separated more than 400 million years Reviewed by: ago. Lycophytes retain many of the features of early vascular plants. Based on genome David Roy Smith, and transcriptome data, we identified WUSCHEL-RELATED HOMEOBOX (WOX) genes University of Western Ontario, Canada Rajib Bandopadhyay, in Selaginella kraussiana, a model lycophyte that is convenient for in vitro culture The University of Burdwan, India and observations of organ formation and regeneration. WOX genes are key players *Correspondence: controlling stem cells in plants. Our results showed that the S. -

New and Noteworthy Additions to the Arkansas Fern Flora
Peck, J.H. 2011. New and noteworthy additions to the Arkansas fern flora. Phytoneuron 2011-30: 1–33. NEW AND NOTEWORTHY ADDITIONS TO THE ARKANSAS FERN FLORA JAMES H. PECK Department Biology University of Arkansas at Little Rock 2801 S. University Ave. Little Rock, AR 72204 [email protected] ABSTRACT Since 1995, 11 fern taxa have been added to the Arkansas flora as new and native, including Asplenium montanum , A. ruta-muraria , A. septentrianale , A. ×trudellii , Athyrium angustum , Azolla caroliniana , Dryopteris goldiana , D. celsa × goldiana , Marsilea macropoda , Palhinhaea cernua , and Trichomanes intracatum . Of the reported Arkansas native ferns, one was deleted (Azolla caroliniana ), being subsumed by Azolla mexicana and now correctly known as Azolla microphylla . Since 1995, 20 fern taxa have been added to the Arkansas fern flora as new and naturalized, including Arachnioides simplicior , Athyrium nipponicum ‘Pictum’, Cyrtomium falcatum , C. fortunei , Dryopteris erythrospora , Hypolepis tenuifolia , Marsilea mutica , M. quadrifolia , Matteuccia struthiopteris , Nephrolepis exaltata , Polystichum tsus-sinense , Phegopteris decursive-pinnata , Salvinia minima , S. molesta , Selaginella braunii , S. kraussiana , S. k. ‘Aurea’, S. k. ‘Brownii’, S. k. ‘Goldtips’, and S. uncinata . Of the reported Arkansas naturalized ferns, one was deleted (C. fortunei ), being without a known voucher. There are now 97 native and 24 naturalized fern taxa known and documented in the Arkansas fern flora. The total Arkansas fern flora is now 121 taxa documented with 3019 county-level occurrence records. Noteworthy update records and comments are reported for 79 of 97 Arkansas native species and 25 Arkansas naturalized species. KEY WORDS : Arkansas, ferns, county distribution Over the last 30 years, studies have been conducted to document the diversity and abundance of the Arkansas fern [pteridophyte] flora. -

81 Vascular Plant Diversity
f 80 CHAPTER 4 EVOLUTION AND DIVERSITY OF VASCULAR PLANTS UNIT II EVOLUTION AND DIVERSITY OF PLANTS 81 LYCOPODIOPHYTA Gleicheniales Polypodiales LYCOPODIOPSIDA Dipteridaceae (2/Il) Aspleniaceae (1—10/700+) Lycopodiaceae (5/300) Gleicheniaceae (6/125) Blechnaceae (9/200) ISOETOPSIDA Matoniaceae (2/4) Davalliaceae (4—5/65) Isoetaceae (1/200) Schizaeales Dennstaedtiaceae (11/170) Selaginellaceae (1/700) Anemiaceae (1/100+) Dryopteridaceae (40—45/1700) EUPHYLLOPHYTA Lygodiaceae (1/25) Lindsaeaceae (8/200) MONILOPHYTA Schizaeaceae (2/30) Lomariopsidaceae (4/70) EQifiSETOPSIDA Salviniales Oleandraceae (1/40) Equisetaceae (1/15) Marsileaceae (3/75) Onocleaceae (4/5) PSILOTOPSIDA Salviniaceae (2/16) Polypodiaceae (56/1200) Ophioglossaceae (4/55—80) Cyatheales Pteridaceae (50/950) Psilotaceae (2/17) Cibotiaceae (1/11) Saccolomataceae (1/12) MARATTIOPSIDA Culcitaceae (1/2) Tectariaceae (3—15/230) Marattiaceae (6/80) Cyatheaceae (4/600+) Thelypteridaceae (5—30/950) POLYPODIOPSIDA Dicksoniaceae (3/30) Woodsiaceae (15/700) Osmundales Loxomataceae (2/2) central vascular cylinder Osmundaceae (3/20) Metaxyaceae (1/2) SPERMATOPHYTA (See Chapter 5) Hymenophyllales Plagiogyriaceae (1/15) FIGURE 4.9 Anatomy of the root, an apomorphy of the vascular plants. A. Root whole mount. B. Root longitudinal-section. C. Whole Hymenophyllaceae (9/600) Thyrsopteridaceae (1/1) root cross-section. D. Close-up of central vascular cylinder, showing tissues. TABLE 4.1 Taxonomic groups of Tracheophyta, vascular plants (minus those of Spermatophyta, seed plants). Classes, orders, and family names after Smith et al. (2006). Higher groups (traditionally treated as phyla) after Cantino et al. (2007). Families in bold are described in found today in the Selaginellaceae of the lycophytes and all the pericycle or endodermis. Lateral roots penetrate the tis detail. -

The Case of Ferns Flora of the Gibraltar Arc
ARTÍCULOS Botanica Complutensis ISSN-e 1988-2874 https://dx.doi.org/10.5209/bocm.75454 The historical biogeography and conservation value of taxonomic distinctness: The case of ferns flora of the Gibraltar Arc Ángel Enrique Salvo Tierra1; José C. Báez2; Antonio Flores-Moya3 Abstract. The pteridofloras of nine locations in the Gibraltar Arc were analyzed using a taxonomic distinctness index. We found that the index could be a proxy of historical biogeography of the pteridofloras from this area. Moreover, the value of the taxonomic distinctness index of the different locations showed relevant relationships with certain geographic variables. Finally, we hypothesize about the value of the information derived from taxonomic distinctness index for conservation of the pteridoflora in the GibraltarArc. Keywords: Pteridophytes, Western Mediterranean, Historical biogeography, Conservation [es] Biogeografía histórica y valor conservacionista de la diferenciación taxonómica: ejemplo de la pteriflora de Arco de Gibraltar Resumen. La pteridoflora de nueve localidades singulares situadas en el Arco de Gibraltar ha sido analizada utilizando un índice de distinción taxonómica. Encontramos que el índice podría ser una aproximación a la historia biogeográfica de las pteridofloras de esta zona. Además, el valor del índice de distinción taxonómica de las diferentes localidades mostró relaciones relevantes con determinadas variables geográficas. Finalmente, discutimos el posible papel de la información derivada del índice de distinción taxonómica en la conservación