Clase 9 Magnoliidae-2015.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Apiales, Aquifoliales, Boraginales, , Brassicales, Canellales
Kingdom: Plantae Phylum: Tracheophyta Class: Magnoliopsida Order: Apiales, Aquifoliales, Boraginales, , Brassicales, Canellales, Caryophyllales, Celastrales, Ericales, Fabales, Garryales, Gentianales, Lamiales, Laurales, Magnoliales, Malpighiales, Malvales, Myrtales, Oxalidales, Picramniales, Piperales, Proteales, Rosales, Santalales, Sapindales, Solanales Family: Achariaceae, Anacardiaceae, Annonaceae, Apocynaceae, Aquifoliaceae, Araliaceae, Bignoniaceae, Bixaceae, Boraginaceae, Burseraceae, Calophyllaceae, Canellaceae, Cannabaceae, Capparaceae, Cardiopteridaceae, Caricaceae, Caryocaraceae, Celastraceae, Chrysobalanaceae, Clusiaceae, Combretaceae, Dichapetalaceae, Ebenaceae, Elaeocarpaceae, Emmotaceae, Erythroxylaceae, Euphorbiaceae, Fabaceae, Goupiaceae, Hernandiaceae, Humiriaceae, Hypericaceae, Icacinaceae, Ixonanthaceae, Lacistemataceae, Lamiaceae, Lauraceae, Lecythidaceae, Lepidobotryaceae, Linaceae, Loganiaceae, Lythraceae, Malpighiaceae, Malvaceae, Melastomataceae, Meliaceae, Monimiaceae, Moraceae, Myristicaceae, Myrtaceae, Nyctaginaceae, Ochnaceae, Olacaceae, Oleaceae, Opiliaceae, Pentaphylacaceae, Phyllanthaceae, Picramniaceae, Piperaceae, Polygonaceae, Primulaceae, Proteaceae, Putranjivaceae, Rhabdodendraceae, Rhamnaceae, Rhizophoraceae, Rosaceae, Rubiaceae, Rutaceae, Sabiaceae, Salicaceae, Sapindaceae, Sapotaceae, Simaroubaceae, Siparunaceae, Solanaceae, Stemonuraceae, Styracaceae, Symplocaceae, Ulmaceae, Urticaceae, Verbenaceae, Violaceae, Vochysiaceae Genus: Abarema, Acioa, Acosmium, Agonandra, Aiouea, Albizia, Alchornea, -

Well-Known Plants in Each Angiosperm Order
Well-known plants in each angiosperm order This list is generally from least evolved (most ancient) to most evolved (most modern). (I’m not sure if this applies for Eudicots; I’m listing them in the same order as APG II.) The first few plants are mostly primitive pond and aquarium plants. Next is Illicium (anise tree) from Austrobaileyales, then the magnoliids (Canellales thru Piperales), then monocots (Acorales through Zingiberales), and finally eudicots (Buxales through Dipsacales). The plants before the eudicots in this list are considered basal angiosperms. This list focuses only on angiosperms and does not look at earlier plants such as mosses, ferns, and conifers. Basal angiosperms – mostly aquatic plants Unplaced in order, placed in Amborellaceae family • Amborella trichopoda – one of the most ancient flowering plants Unplaced in order, placed in Nymphaeaceae family • Water lily • Cabomba (fanwort) • Brasenia (watershield) Ceratophyllales • Hornwort Austrobaileyales • Illicium (anise tree, star anise) Basal angiosperms - magnoliids Canellales • Drimys (winter's bark) • Tasmanian pepper Laurales • Bay laurel • Cinnamon • Avocado • Sassafras • Camphor tree • Calycanthus (sweetshrub, spicebush) • Lindera (spicebush, Benjamin bush) Magnoliales • Custard-apple • Pawpaw • guanábana (soursop) • Sugar-apple or sweetsop • Cherimoya • Magnolia • Tuliptree • Michelia • Nutmeg • Clove Piperales • Black pepper • Kava • Lizard’s tail • Aristolochia (birthwort, pipevine, Dutchman's pipe) • Asarum (wild ginger) Basal angiosperms - monocots Acorales -

Heterodichogamy.Pdf
Research Update TRENDS in Ecology & Evolution Vol.16 No.11 November 2001 595 How common is heterodichogamy? Susanne S. Renner The sexual systems of plants usually Heterodichogamy differs from normal (Zingiberales). These figures probably depend on the exact spatial distribution of dichogamy, the temporal separation of underestimate the frequency of the gamete-producing structures. Less well male and female function in flowers, in heterodichogamy. First, the phenomenon known is how the exact timing of male and that it involves two genetic morphs that is discovered only if flower behavior is female function might influence plant occur at a 1:1 ratio. The phenomenon was studied in several individuals and in mating. New papers by Li et al. on a group discovered in walnuts and hazelnuts5,6 natural populations. Differential of tropical gingers describe differential (the latter ending a series of Letters to movements and maturation of petals, maturing of male and female structures, the Editor about hazel flowering that styles, stigmas and stamens become such that half the individuals of a began in Nature in 1870), but has gone invisible in dried herbarium material, population are in the female stage when almost unnoticed7. Indeed, its recent and planted populations deriving from the other half is in the male stage. This discovery in Alpinia was greeted as a vegetatively propagated material no new case of heterodichogamy is unique new mechanism, differing ‘from other longer reflect natural morph ratios. The in involving reciprocal movement of the passive outbreeding devices, such as discovery of heterodichogamy thus styles in the two temporal morphs. dichogamy…and heterostyly in that it depends on field observations. -

Phylogenomic Approach
Toward the ultimate phylogeny of Magnoliaceae: phylogenomic approach Sangtae Kim*1, Suhyeon Park1, and Jongsun Park2 1 Sungshin University, Korea 2 InfoBoss Co., Korea Mr. Carl Ferris Miller Founder of Chollipo Arboretum in Korea Chollipo Arboretum Famous for its magnolia collection 2020. Annual Meeting of Magnolia Society International Cholliop Arboretum in Korea. April 13th~22th, 2020 http://WWW.Chollipo.org Sungshin University, Seoul, Korea Dr. Hans Nooteboom Dr. Liu Yu-Hu Twenty-one years ago... in 1998 The 1st International Symposium on the Family Magnoliaceae, Gwangzhow Dr. Hiroshi Azuma Mr. Richard Figlar Dr. Hans Nooteboom Dr. Qing-wen Zeng Dr. Weibang Sun Handsome young boy Dr. Yong-kang Sima Dr. Yu-wu Law Presented ITS study on Magnoliaceae - never published Ten years ago... in 2009 Presented nine cp genome region study (9.2 kbp) on Magnoliaceae – published in 2013 2015 1st International Sympodium on Neotropical Magnoliaceae Gadalajara, 2019 3rd International Sympodium and Workshop on Neotropical Magnoliaceae Asterales Dipsacales Apiales Why magnolia study is Aquifoliales Campanulids (Euasterids II) Garryales Gentianales Laminales Solanales Lamiids important in botany? Ericales Asterids (Euasterids I) Cornales Sapindales Malvales Brassicales Malvids Fagales (Eurosids II) • As a member of early-diverging Cucurbitales Rosales Fabales Zygophyllales Celestrales Fabids (Eurosid I) angiosperms, reconstruction of the Oxalidales Malpighiales Vitales Geraniales Myrtales Rosids phylogeny of Magnoliaceae will Saxifragales Caryphyllales -

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time. -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

Piperaceae) Revealed by Molecules
Annals of Botany 99: 1231–1238, 2007 doi:10.1093/aob/mcm063, available online at www.aob.oxfordjournals.org From Forgotten Taxon to a Missing Link? The Position of the Genus Verhuellia (Piperaceae) Revealed by Molecules S. WANKE1 , L. VANDERSCHAEVE2 ,G.MATHIEU2 ,C.NEINHUIS1 , P. GOETGHEBEUR2 and M. S. SAMAIN2,* 1Technische Universita¨t Dresden, Institut fu¨r Botanik, D-01062 Dresden, Germany and 2Ghent University, Department of Biology, Research Group Spermatophytes, B-9000 Ghent, Belgium Downloaded from https://academic.oup.com/aob/article/99/6/1231/2769300 by guest on 28 September 2021 Received: 6 December 2006 Returned for revision: 22 January 2007 Accepted: 12 February 2007 † Background and Aims The species-poor and little-studied genus Verhuellia has often been treated as a synonym of the genus Peperomia, downplaying its significance in the relationships and evolutionary aspects in Piperaceae and Piperales. The lack of knowledge concerning Verhuellia is largely due to its restricted distribution, poorly known collection localities, limited availability in herbaria and absence in botanical gardens and lack of material suitable for molecular phylogenetic studies until recently. Because Verhuellia has some of the most reduced flowers in Piperales, the reconstruction of floral evolution which shows strong trends towards reduction in all lineages needs to be revised. † Methods Verhuellia is included in a molecular phylogenetic analysis of Piperales (trnT-trnL-trnF and trnK/matK), based on nearly 6000 aligned characters and more than 1400 potentially parsimony-informative sites which were partly generated for the present study. Character states for stamen and carpel number are mapped on the combined molecular tree to reconstruct the ancestral states. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M. -

Updated Angiosperm Family Tree for Analyzing Phylogenetic Diversity and Community Structure
Acta Botanica Brasilica - 31(2): 191-198. April-June 2017. doi: 10.1590/0102-33062016abb0306 Updated angiosperm family tree for analyzing phylogenetic diversity and community structure Markus Gastauer1,2* and João Augusto Alves Meira-Neto2 Received: August 19, 2016 Accepted: March 3, 2017 . ABSTRACT Th e computation of phylogenetic diversity and phylogenetic community structure demands an accurately calibrated, high-resolution phylogeny, which refl ects current knowledge regarding diversifi cation within the group of interest. Herein we present the angiosperm phylogeny R20160415.new, which is based on the topology proposed by the Angiosperm Phylogeny Group IV, a recently released compilation of angiosperm diversifi cation. R20160415.new is calibratable by diff erent sets of recently published estimates of mean node ages. Its application for the computation of phylogenetic diversity and/or phylogenetic community structure is straightforward and ensures the inclusion of up-to-date information in user specifi c applications, as long as users are familiar with the pitfalls of such hand- made supertrees. Keywords: angiosperm diversifi cation, APG IV, community tree calibration, megatrees, phylogenetic topology phylogeny comprising the entire taxonomic group under Introduction study (Gastauer & Meira-Neto 2013). Th e constant increase in knowledge about the phylogenetic The phylogenetic structure of a biological community relationships among taxa (e.g., Cox et al. 2014) requires regular determines whether species that coexist within a given revision of applied phylogenies in order to incorporate novel data community are more closely related than expected by chance, and is essential information for investigating and avoid out-dated information in analyses of phylogenetic community assembly rules (Kembel & Hubbell 2006; diversity and community structure. -

Phylogeny, Molecular Dating, and Floral Evolution of Magnoliidae (Angiospermae)
UNIVERSITÉ PARIS-SUD ÉCOLE DOCTORALE : SCIENCES DU VÉGÉTAL Laboratoire Ecologie, Systématique et Evolution DISCIPLINE : BIOLOGIE THÈSE DE DOCTORAT Soutenue le 11/04/2014 par Julien MASSONI Phylogeny, molecular dating, and floral evolution of Magnoliidae (Angiospermae) Composition du jury : Directeur de thèse : Hervé SAUQUET Maître de Conférences (Université Paris-Sud) Rapporteurs : Susanna MAGALLÓN Professeur (Universidad Nacional Autónoma de México) Thomas HAEVERMANS Maître de Conférences (Muséum national d’Histoire Naturelle) Examinateurs : Catherine DAMERVAL Directeur de Recherche (CNRS, INRA) Michel LAURIN Directeur de Recherche (CNRS, Muséum national d’Histoire Naturelle) Florian JABBOUR Maître de Conférences (Muséum national d’Histoire Naturelle) Michael PIRIE Maître de Conférences (Johannes Gutenberg Universität Mainz) Membres invités : Hervé SAUQUET Maître de Conférences (Université Paris-Sud) Remerciements Je tiens tout particulièrement à remercier mon directeur de thèse et ami Hervé Sauquet pour son encadrement, sa gentillesse, sa franchise et la confiance qu’il m’a accordée. Cette relation a immanquablement contribuée à ma progression humaine et scientifique. La pratique d’une science sans frontière est la plus belle chose qu’il m’ait apportée. Ce fut enthousiasmant, très fructueux, et au-delà de mes espérances. Ce mode de travail sera le mien pour la suite de ma carrière. Je tiens également à remercier ma copine Anne-Louise dont le soutien immense a contribué à la réalisation de ce travail. Elle a vécu avec patience et attention les moments d’enthousiasmes et de doutes. Par la même occasion, je remercie ma fille qui a eu l’heureuse idée de ne pas naître avant la fin de la rédaction de ce manuscrit. -
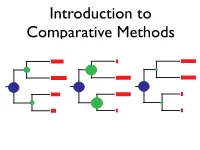
BM CC EB What Can We Learn from a Tree?
Introduction to Comparative Methods BM CC EB What can we learn from a tree? Net diversification (r) Relative extinction (ε) Peridiscaceae Peridiscaceae yllaceae yllaceae h h atop atop Proteaceae Proteaceae r r Ce Ce Tr oc T ho r M M o de c y y H H C C h r r e e a D D a o o nd o e e G G a a m m t t a a d r P r P h h e e u u c c p A p A r e a a e e a a a c a c n n a i B i B h h n d m d l m a l a m m e a e a e e t t n n c u u n n i i d i i e e e e o n o n p n p n a e a S e e S e e n n x x i i r c c a a n o n o p p h g e h g ae e l r a l r a a a a a a i i a a a e a e i b i b h y d c h d c i y i c a a c x c x c c G I a G I a n c n c c c y l y l t a a t a a e e e e e i l c i l c m l m l e c e c f a e a a f a e a a l r r l c c a i i r l e t e t a a r l a a e e u u u u o a o a a a c a c a a l a l e e e b b a a a a e e c e e c a a s c s c c e l c e l e e g e g e a a a a e e n n s e e s e e e e a a a P a P e e N N u u u S u S a e a e a a e e c c l n a l n e e a e e a e a e a e a e r a r a c c C i C i R R a e a e a e a e r c r c A A a d a a d a e i e i phanopetalaceae s r e ph s r e a a s e c s e c e e u u b a a b a e e P P r r l l e n e a a a a m m entho e e e Ha a H o a c r e c r e nt B B e p e e e c e e c a c c h e a p a a p a lo lo l l a a e s o t e s i a r a i a r r r r r a n e a n e a b a l b t a t gaceae e g e ceae a c a s c s a z e z M i a e M i a c a d e a d e ae e ae r e r a e a e a a a c c ce r e r L L i i ac a Vitaceae Vi r r C C e e ta v e v e a a c a a e ea p e ap c a c a e a P P e e l l e Ge G e e ae a t t e e p p r r ce c an u an -

Field Identification of the 50 Most Common Plant Families in Temperate Regions
Field identification of the 50 most common plant families in temperate regions (including agricultural, horticultural, and wild species) by Lena Struwe [email protected] © 2016, All rights reserved. Note: Listed characteristics are the most common characteristics; there might be exceptions in rare or tropical species. This compendium is available for free download without cost for non- commercial uses at http://www.rci.rutgers.edu/~struwe/. The author welcomes updates and corrections. 1 Overall phylogeny – living land plants Bryophytes Mosses, liverworts, hornworts Lycophytes Clubmosses, etc. Ferns and Fern Allies Ferns, horsetails, moonworts, etc. Gymnosperms Conifers, pines, cycads and cedars, etc. Magnoliids Monocots Fabids Ranunculales Rosids Malvids Caryophyllales Ericales Lamiids The treatment for flowering plants follows the APG IV (2016) Campanulids classification. Not all branches are shown. © Lena Struwe 2016, All rights reserved. 2 Included families (alphabetical list): Amaranthaceae Geraniaceae Amaryllidaceae Iridaceae Anacardiaceae Juglandaceae Apiaceae Juncaceae Apocynaceae Lamiaceae Araceae Lauraceae Araliaceae Liliaceae Asphodelaceae Magnoliaceae Asteraceae Malvaceae Betulaceae Moraceae Boraginaceae Myrtaceae Brassicaceae Oleaceae Bromeliaceae Orchidaceae Cactaceae Orobanchaceae Campanulaceae Pinaceae Caprifoliaceae Plantaginaceae Caryophyllaceae Poaceae Convolvulaceae Polygonaceae Cucurbitaceae Ranunculaceae Cupressaceae Rosaceae Cyperaceae Rubiaceae Equisetaceae Rutaceae Ericaceae Salicaceae Euphorbiaceae Scrophulariaceae