Project Bioshield: Appropriations, Acquisitions, and Policy Implementation Issues for Congress
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

ANTHRASIL™ Safely and Effectively
53 Weight-based Pediatric Dose Body Weight Vials per Dosea Body Weight Vials per Dose (kg) (kg) 1 HIGHLIGHTS OF PRESCRIBING INFORMATION <5 1 25 to <35 4 <10 1 35 to <50 5 10 to <18 2 50 to <60 6 These highlights do not include all the information needed to use 2 18 to <25 3 ≥60 7 ANTHRASIL™ safely and effectively. See full prescribing information for 3 aSelect initial dose based on clinical severity. Dose may be doubled for severe ANTHRASIL. 4 cases in patients >5 kg. 5 ANTHRASIL [Anthrax Immune Globulin Intravenous (Human)], sterile 54 6 Administer ANTHRASIL by slow intravenous infusion using an infusion solution for infusion 55 7 pump (maximum 2 mL per minute). 56 8 Initial U.S. Approval: March 24, 2015 57 9 ---------------------DOSAGE FORMS AND STRENGTHS---------------------- 10 58 59 Each single-use vial contains a minimum potency of ≥60 units by Toxin 11 WARNING: INTERACTIONS WITH GLUCOSE MONITORING 60 Neutralization Assay (TNA) (3). 12 SYSTEMS AND THROMBOSIS 61 13 See full prescribing information for complete boxed warning. 62 -------------------------------CONTRAINDICATIONS------------------------------ 14 • Maltose in immune globulin products, including ANTHRASIL, may give 63 • History of anaphylactic or severe systemic reaction to human immune 15 falsely high blood glucose levels with some blood point-of-care glucose 64 globulins (4) 16 testing systems (for example those based on the GDH-PQQ or glucose-dye- 65 • IgA deficiency with antibodies against IgA and a history of IgA 17 oxidoreductase methods) resulting in inappropriate administration of insulin 66 hypersensitivity (4) 18 and life-threatening hypoglycemia. To avoid interference by maltose 67 19 contained in ANTHRASIL, perform blood glucose measurements in patients 68 -----------------------WARNINGS AND PRECAUTIONS------------------------ 20 receiving ANTHRASIL with a glucose-specific method (monitor and test 21 strips). -

UN Health Update #58
UN Health Update #58 Multilateral Consulting, LLC November 1, 2017 UN HEALTH UPDATE #58 NOVEMBER 1, 2017 Table of Contents WHO Breaks Precedent with New Leadership Team ............................................... 1 Female majority, inclusion of conservatives in line-up WIPO Showcases Agency’s Work, Highlights IFPMA Project .................................. 5 At Assemblies: committees report, "Pat-INFORMED" presented “Montevideo Roadmap” Issued by Leaders at WHO NCD Confab .......................... 8 High-level attendance at October Uruguay meeting WHO Establishes High-Level NCD Commission ..................................................... 9 Headed by Sania Nishtar, will include Peruvian president WHO Issues GCM/NCD Progress Report ............................................................... 9 Aims to achieve Global Monitoring Framework's 9 targets WHO EURO, LSE Scholars: Hurdles are Prices, IP ............................................... 10 Slams failure to implement HLP recommencations, including "punishment' Comments Submitted on WHO GPW 2019-2023 ................................................... 10 Lays out D-G's vision and priorities; NGOs make suggestions PAHO Elects Carissa Etienne to 2nd Term .............................................................. 12 Directing Council takes up usual list of topics Regional Director Presides Over First/Last EMRO Meeting .................................. 12 Ministers debate range of pressing challenges WPRO Takes Up Broad Range of Issues ............................................................... -

The Effect of Raxibacumab on the Immunogenicity of Anthrax Vaccine Adsorbed: a Phase
The effect of raxibacumab on the immunogenicity of anthrax vaccine adsorbed: a Phase IV, randomised, open-label, parallel-group, non-inferiority study Nancy Skoura, PhD1, Jie Wang-Jairaj, MD2, Oscar Della Pasqua MD2, Vijayalakshmi Chandrasekaran, MS1, Julia Billiard, PhD1, Anne Yeakey, MD3, William Smith, MD4, Helen Steel, MD2, Lionel K Tan, FRCP2 1GlaxoSmithKline, Inc. Collegeville, PA, USA 2GlaxoSmithKline. Stockley Park West, Middlesex, UK 3GlaxoSmithKline, Inc. Rockville, MD, USA 4AMR, at University of TN Medical Center, Knoxville, TN; New Orleans Center for Clinical Research (NOCCR), USA Author for Correspondence: Dr Lionel K Tan GlaxoSmithKline Stockley Park West 1–3 Ironbridge Road Uxbridge Middlesex UB11 1BT UK Email: [email protected] Tel: +44 (0)7341 079 683 1 Abstract: 340/350 Body text: 4321/4500 words including Research in Context Table/Figures: 2/4 References: 30 2 Abstract Background Raxibacumab is a monoclonal antibody (Ab) which binds protective antigen (PA) of Bacillus anthracis and is approved for treatment and post-exposure prophylaxis (PEP) of inhalational anthrax. Anthrax vaccine adsorbed (AVA), for anthrax prophylaxis, consists primarily of adsorbed PA. This post-approval study evaluated the effect of raxibacumab on immunogenicity of AVA. Methods In this open-label, parallel-group, non-inferiority study in three centres in the USA, healthy volunteers (aged 18–65 years) with no evidence of PA pre-exposure were randomised 1:1 to receive either subcutaneous 0·5 mL AVA on Days 1, 15, and 29 or raxibacumab intravenous infusion (40 mg/kg) immediately before AVA on Day 1, followed by AVA only on Days 15 and 29. -

Acquisitions, and Policy Implementation Issues for Congress
Order Code RL33907 Project BioShield: Appropriations, Acquisitions, and Policy Implementation Issues for Congress March 8, 2007 Frank Gottron Specialist in Science and Technology Policy Resources, Science, and Industry Division Project BioShield: Appropriations, Acquisitions, and Policy Implementation Issues for Congress Summary The Project BioShield Act of 2004 (P.L. 108-276) established a 10-year program to acquire civilian medical countermeasures to chemical, biological, radiological and nuclear (CBRN) agents for the Strategic National Stockpile. Provisions of this act were designed to encourage private companies to develop these countermeasures by guaranteeing a government market for successfully developed countermeasures. Both the Department of Homeland Security (DHS) and the Department of Health and Human Services (HHS) have responsibilities in this program. Funds for this program are appropriated to DHS, while contracts are executed through HHS. The interagency process responsible for deciding which countermeasures to procure has changed multiple times since this program’s inception. The Homeland Security Appropriations Act, 2004 (P.L. 108-90) provided an advance appropriation of $5.6 billion to acquire CBRN countermeasures over a 10- year period (FY2004–FY2013). This act also limited the amount that could be obligated during specified time periods. The Project BioShield Act of 2004 (P.L. 108-276) assigned the $5.6 billion advance appropriation to Project BioShield countermeasure acquisitions. The Consolidated Appropriations Act, 2004 (P.L. 108- 199) and the Consolidated Appropriations Act, 2005 (P.L. 108-447) reduced the total amount available for Project BioShield by a total of $25 million. Congress retains the power to make additional appropriations and rescissions to this account. -

Avian Influenza Pandemic May Expand the Military Role in Disaster Relief
USAWC STRATEGY RESEARCH PROJECT AVIAN INFLUENZA PANDEMIC MAY EXPAND THE MILITARY ROLE IN DISASTER RELIEF by Colonel Frank William Sherod II United States Army Colonel Dallas C. Hack Project Adviser This SRP is submitted in partial fulfillment of the requirements of the Master of Strategic Studies Degree. The U.S. Army War College is accredited by the Commission on Higher Education of the Middle States Association of Colleges and Schools, 3624 Market Street, Philadelphia, PA 19104, (215) 662-5606. The Commission on Higher Education is an institutional accrediting agency recognized by the U.S. Secretary of Education and the Council for Higher Education Accreditation. The views expressed in this student academic research paper are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government. U.S. Army War College CARLISLE BARRACKS, PENNSYLVANIA 17013 Form Approved Report Documentation Page OMB No. 0704-0188 Public reporting burden for the collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to Washington Headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington VA 22202-4302. Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to a penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. -

A Review of the Efficacy of FDA-Approved B. Anthracis Anti
toxins Review A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models Zoe Couse 1, Xizhong Cui 1, Yan Li 1, Mahtab Moayeri 2, Stephen Leppla 2 and Peter Q. Eichacker 1,* 1 Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (Z.C.); [email protected] (X.C.); [email protected] (Y.L.) 2 National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.M.); [email protected] (S.L.) * Correspondence: [email protected] Abstract: Anti-toxin agents for severe B. anthracis infection will only be effective if they add to the benefit of the two mainstays of septic shock management, antibiotic therapy and titrated hemody- namic support. Both of these standard therapies could negate benefits related to anti-toxin treatment. At present, three anthrax anti-toxin antibody preparations have received US Food and Drug Adminis- tration (FDA) approval: Raxibacumab, Anthrax Immune Globulin Intravenous (AIGIV) and ETI-204. Each agent is directed at the protective antigen component of lethal and edema toxin. All three agents were compared to placebo in antibiotic-treated animal models of live B. anthracis infection, and Raxibacumab and AIGIV were compared to placebo when combined with standard hemodynamic support in a 96 h canine model of anthrax toxin-associated shock. However, only AIG has actually Citation: Couse, Z.; Cui, X.; Li, Y.; been administered to a group of infected patients, and this experience was not controlled and offers Moayeri, M.; Leppla, S.; Eichacker, P.Q. -

The Project Bioshield Act: Issues for the 112Th Congress
The Project BioShield Act: Issues for the 112th Congress Frank Gottron Specialist in Science and Technology Policy March 13, 2012 Congressional Research Service 7-5700 www.crs.gov R42349 CRS Report for Congress Prepared for Members and Committees of Congress The Project BioShield Act: Issues for the 112th Congress Summary In 2004, Congress passed the Project BioShield Act (P.L. 108-276) to provide the federal government with new authorities related to the development, procurement, and use of medical countermeasures against chemical, biological, radiological, and nuclear (CBRN) terrorism agents. As the expiration of some of these authorities approaches, Congress is considering whether these authorities have sufficiently contributed to national preparedness to merit extension. The Project BioShield Act provides three main authorities: (1) guaranteeing a federal market for new CBRN medical countermeasures, (2) permitting emergency use of countermeasures that are either unapproved or have not been approved for the intended emergency use, and (3) relaxing regulatory requirements for some CBRN terrorism-related spending. The Department of Health and Human Services (HHS) has used each of these authorities. The HHS obligated approximately $2.5 billion to guarantee a government market for countermeasures against anthrax, botulism, radiation, and smallpox. The HHS allowed the emergency use of several unapproved products, including during the 2009 H1N1 influenza pandemic. The HHS used expedited review authorities to approve contracts and grants related to CBRN countermeasure research and development. The Department of Homeland Security (DHS) Appropriations Act, 2004 (P.L. 108-90) advance- appropriated $5.593 billion to acquire CBRN countermeasures through Project BioShield for FY2004-FY2013. Through FY2012, subsequent Congresses have removed $1.876 billion from this account through rescissions and transfers, more than one-third of the advance appropriation. -

It's Getting Ugly out There
It’s Getting Ugly Out There The Frauds, Bunglers, Liars, and Losers Who Are Hurting America JACK CAFFERTY John Wiley & Sons, Inc. It’s Getting Ugly Out There It’s Getting Ugly Out There The Frauds, Bunglers, Liars, and Losers Who Are Hurting America JACK CAFFERTY John Wiley & Sons, Inc. Copyright © 2007 by Jack Cafferty. All rights reserved Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada Design and composition by Navta Associates, Inc. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copy- right Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permissions. Limit of Liability/Disclaimer of Warranty: While the publisher and the author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically dis- claim any implied warranties of merchantability or fitness for a particular purpose. -
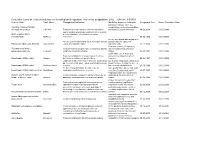
Designated Indication Cumulative List of All Products That Have Received
Cumulative List of all Products that have received Orphan Designation: Total active designations: 2002 Effecive: 5/5/2009 Generic Name Trade Name Designated Indication treatmentMarketing and/or Approved modification Indication of the Designated Date Market Exculsivity Date following conditions, which are Vaccinia Immune Globulin complications resulting from smallpox (Human) Intravenous CNJ-016 ForTreatment the control of complications and prevention of vacciniaof hemorrhagic vaccination episodes vaccination: Eczema vaccinatum 18.06.2004 01.05.9999 and for surgical prophylaxis in patients with hemophilia Antihemophilic factor A (congenital factor VIII deficiency or classic (recombinant) ReFacto hemophilia). 08.02.1996 01.01.9999 For use as a thyroid blocking agent in For use as a thyroid blocking agent in pediatric patients pediatric patients exposed to Potassium Iodide Oral Solution ThyroShield exposed to radiactive iodine radiactive iodine 17.11.2004 01.01.9999 Treatment (rescue) of respiratory Pulmonary surfactant For the treatment and prevention of respiratory distress distress syndrome in premature replacement, porcine Curosurf syndrome in premature infants. Longinfants. term treatment of children with 02.08.1993 01.01.9999 growth failure due to inadequate Treatment of idiopathic or organic growth hormone secretion of endogenous growth Somatropin (rDNA origin) Saizen deficiency in children with growth failure. Long-termhormone. treatment of growth failure 06.03.1987 01.01.9999 Long-term treatment of children who have growth failure due to a lack of adequate endogenous due to a lack of adequate endogenous growth hormone growth hormone secetion for once- or Somatropin (rDNA origin) Nutropin Depot secretion. twice-a-month administration. 28.10.1999 01.01.9999 Treatment of growth failure in children due to have growth failure due to inadequate Somatropin (rDNA origin) injection Norditropin inadequate growth hormone secretion. -

Ibm Micromedex® Carenotes Titles by Category
IBM MICROMEDEX® CARENOTES TITLES BY CATEGORY DECEMBER 2019 © Copyright IBM Corporation 2019 All company and product names mentioned are used for identification purposes only and may be trademarks of their respective owners. Table of Contents IBM Micromedex® CareNotes Titles by Category Allergy and Immunology ..................................................................................................................2 Ambulatory.......................................................................................................................................3 Bioterrorism ...................................................................................................................................18 Cardiology......................................................................................................................................18 Critical Care ...................................................................................................................................20 Dental Health .................................................................................................................................22 Dermatology ..................................................................................................................................23 Dietetics .........................................................................................................................................24 Endocrinology & Metabolic Disease ..............................................................................................26 -

The Project Bioshield Act: Issues for the 113Th Congress
The Project BioShield Act: Issues for the 113th Congress Frank Gottron Specialist in Science and Technology Policy June 18, 2014 Congressional Research Service 7-5700 www.crs.gov R43607 The Project BioShield Act: Issues for the 113th Congress Summary In 2004, Congress passed the Project BioShield Act (P.L. 108-276) to provide the federal government with new authorities related to the development, procurement, and use of medical countermeasures against chemical, biological, radiological, and nuclear (CBRN) terrorism agents. However, the government still lacks countermeasures against many of the CBRN terrorism agents determined by the government to pose the greatest threat. Congress is likely to consider whether modifications of these authorities or new authorities would help address remaining gaps. The authority generally referred to as Project BioShield allows the government to guarantee a market for CBRN medical countermeasures. Under this provision, the Secretary of Health and Human Services (HHS) may obligate funds to purchase countermeasures that still need up to 10 more years of development. Since 2004, HHS has obligated approximately $3.309 billion to guarantee a government market for countermeasures against anthrax, smallpox, botulism, radiation, and nerve agents. Another provision of the Project BioShield Act established a process through which the HHS Secretary may temporarily allow the emergency use of countermeasures that lack Food and Drug Administration (FDA) approval. The HHS has used this authority to allow the emergency use of unapproved products several times. The Department of Homeland Security (DHS) Appropriations Act, 2004 (P.L. 108-90) advance appropriated $5.593 billion to acquire CBRN countermeasures through Project BioShield between FY2004 and FY2013. -

DTT) Specification Guide
Data Translation Tool (DTT) Specification Guide 7.16.2020 This document provides specific instructions for setting up a file with the correct data elements (fields) for DTT. Table of Contents Permissions ......................................................................................................................................................................................... 4 File Format Elements ....................................................................................................................................................................... 4 Creating a Data File ........................................................................................................................................................................... 4 Import Profile Set Up ........................................................................................................................................................................................ 4 Patient Information ...................................................................................................................................................................................... 5 Patient and Vaccination Information .................................................................................................................................................... 6 Inventory Information ................................................................................................................................................................................