The Project Bioshield Act: Issues for the 113Th Congress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GAO-09-878R Project Bioshield Act: HHS Has Supported Development
United States Government Accountability Office Washington, DC 20548 July 24, 2009 Congressional Committees Subject: Project BioShield Act: HHS Has Supported Development, Procurement, and Emergency Use of Medical Countermeasures to Address Health Threats This report formally transmits the attached briefing in response to section 247d-6c of title 42 of the United States Code. (See the enclosure.) The statute required the Comptroller General to examine the Department of Health and Human Services’ (HHS) support for the development and procurement of and authority for the emergency use of medical countermeasures to address chemical, biological, radiological, and nuclear threats to public health, and provide the results to the congressional committees by July 21, 2009.1 HHS determines priorities for medical countermeasure procurement based on those chemical, biological, radiological, and nuclear agents that have been identified by the Department of Homeland Security as posing a material threat to the U.S. population that could affect national security. We provided the briefing to staff of your committees to satisfy the mandate reporting requirement on July 20, 2009, and July 21, 2009. – – – – – We are sending copies of this report to the Secretary of HHS, the Secretary of Homeland Security, and other interested parties. In addition, the report will be available at no charge on the GAO Web site at http://www.gao.gov. If you or your staff have any questions regarding this report, please contact me at (202) 512- 7114 or [email protected]. Contact points for our Offices of Congressional Relations and Public Affairs may be found on the last page of this report. -

Project Bioshield ANNUAL REPORT to CONGRESS AUGUST 2006 – JULY 2007
Project BioShield ANNUAL REPORT TO CONGRESS AUGUST 2006 – JULY 2007 United States Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response Biomedical Advanced Research and Development Authority United States Department of Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response (ASPR) Biomedical Advanced Research and Development Authority (BARDA) Project BioShield Annual Report to Congress August 2006 – July 2007 Contents List of Figures ...................................................................................................................................................... 3 List of Tables ........................................................................................................................................................ 3 1.0 Executive Summary ..................................................................................................................................... 4 2.0 Introduction .................................................................................................................................................. 7 2.1 Historical Background ............................................................................................................................... 7 2.2 Summary of BioShield Authorities and Statutory Reporting Requirements ........................................ 10 2.3 Scope of This Report .............................................................................................................................. -

ANTHRASIL™ Safely and Effectively
53 Weight-based Pediatric Dose Body Weight Vials per Dosea Body Weight Vials per Dose (kg) (kg) 1 HIGHLIGHTS OF PRESCRIBING INFORMATION <5 1 25 to <35 4 <10 1 35 to <50 5 10 to <18 2 50 to <60 6 These highlights do not include all the information needed to use 2 18 to <25 3 ≥60 7 ANTHRASIL™ safely and effectively. See full prescribing information for 3 aSelect initial dose based on clinical severity. Dose may be doubled for severe ANTHRASIL. 4 cases in patients >5 kg. 5 ANTHRASIL [Anthrax Immune Globulin Intravenous (Human)], sterile 54 6 Administer ANTHRASIL by slow intravenous infusion using an infusion solution for infusion 55 7 pump (maximum 2 mL per minute). 56 8 Initial U.S. Approval: March 24, 2015 57 9 ---------------------DOSAGE FORMS AND STRENGTHS---------------------- 10 58 59 Each single-use vial contains a minimum potency of ≥60 units by Toxin 11 WARNING: INTERACTIONS WITH GLUCOSE MONITORING 60 Neutralization Assay (TNA) (3). 12 SYSTEMS AND THROMBOSIS 61 13 See full prescribing information for complete boxed warning. 62 -------------------------------CONTRAINDICATIONS------------------------------ 14 • Maltose in immune globulin products, including ANTHRASIL, may give 63 • History of anaphylactic or severe systemic reaction to human immune 15 falsely high blood glucose levels with some blood point-of-care glucose 64 globulins (4) 16 testing systems (for example those based on the GDH-PQQ or glucose-dye- 65 • IgA deficiency with antibodies against IgA and a history of IgA 17 oxidoreductase methods) resulting in inappropriate administration of insulin 66 hypersensitivity (4) 18 and life-threatening hypoglycemia. To avoid interference by maltose 67 19 contained in ANTHRASIL, perform blood glucose measurements in patients 68 -----------------------WARNINGS AND PRECAUTIONS------------------------ 20 receiving ANTHRASIL with a glucose-specific method (monitor and test 21 strips). -

The Effect of Raxibacumab on the Immunogenicity of Anthrax Vaccine Adsorbed: a Phase
The effect of raxibacumab on the immunogenicity of anthrax vaccine adsorbed: a Phase IV, randomised, open-label, parallel-group, non-inferiority study Nancy Skoura, PhD1, Jie Wang-Jairaj, MD2, Oscar Della Pasqua MD2, Vijayalakshmi Chandrasekaran, MS1, Julia Billiard, PhD1, Anne Yeakey, MD3, William Smith, MD4, Helen Steel, MD2, Lionel K Tan, FRCP2 1GlaxoSmithKline, Inc. Collegeville, PA, USA 2GlaxoSmithKline. Stockley Park West, Middlesex, UK 3GlaxoSmithKline, Inc. Rockville, MD, USA 4AMR, at University of TN Medical Center, Knoxville, TN; New Orleans Center for Clinical Research (NOCCR), USA Author for Correspondence: Dr Lionel K Tan GlaxoSmithKline Stockley Park West 1–3 Ironbridge Road Uxbridge Middlesex UB11 1BT UK Email: [email protected] Tel: +44 (0)7341 079 683 1 Abstract: 340/350 Body text: 4321/4500 words including Research in Context Table/Figures: 2/4 References: 30 2 Abstract Background Raxibacumab is a monoclonal antibody (Ab) which binds protective antigen (PA) of Bacillus anthracis and is approved for treatment and post-exposure prophylaxis (PEP) of inhalational anthrax. Anthrax vaccine adsorbed (AVA), for anthrax prophylaxis, consists primarily of adsorbed PA. This post-approval study evaluated the effect of raxibacumab on immunogenicity of AVA. Methods In this open-label, parallel-group, non-inferiority study in three centres in the USA, healthy volunteers (aged 18–65 years) with no evidence of PA pre-exposure were randomised 1:1 to receive either subcutaneous 0·5 mL AVA on Days 1, 15, and 29 or raxibacumab intravenous infusion (40 mg/kg) immediately before AVA on Day 1, followed by AVA only on Days 15 and 29. -

Project Bioshield Act of 2004
PUBLIC LAW 108–276—JULY 21, 2004 118 STAT. 835 Public Law 108–276 108th Congress An Act To amend the Public Health Service Act to provide protections and countermeasures against chemical, radiological, or nuclear agents that may be used in a terrorist attack against the United States by giving the National Institutes of Health July 21, 2004 contracting flexibility, infrastructure improvements, and expediting the scientific [S. 15] peer review process, and streamlining the Food and Drug Administration approval process of countermeasures. Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, Project BioShield Act of 2004. SECTION 1. SHORT TITLE. 42 USC 201 note. This Act may be cited as the ‘‘Project BioShield Act of 2004’’. SEC. 2. BIOMEDICAL COUNTERMEASURE RESEARCH AND DEVELOP- MENT—AUTHORITIES. (a) IN GENERAL.—Part B of title III of the Public Health Service Act (42 U.S.C. 243 et seq.) is amended by inserting after section 319F the following section: ‘‘SEC. 319F–1. AUTHORITY FOR USE OF CERTAIN PROCEDURES 42 USC 247d–6a. REGARDING QUALIFIED COUNTERMEASURE RESEARCH AND DEVELOPMENT ACTIVITIES. ‘‘(a) IN GENERAL.— ‘‘(1) AUTHORITY.—In conducting and supporting research and development activities regarding countermeasures under section 319F(h), the Secretary may conduct and support such activities in accordance with this section and, in consultation with the Director of the National Institutes of Health, as part of the program under section 446, if the activities concern qualified countermeasures. ‘‘(2) QUALIFIED COUNTERMEASURE.—For purposes of this section, the term ‘qualified countermeasure’ means a drug (as that term is defined by section 201(g)(1) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. -

Biosecurity: a Comprehensive Action Plan Center for American Progress
Center for American Progress Biosecurity A Comprehensive Action Plan Andrew J. Grotto Jonathan B. Tucker Progressive Ideas for a Strong, Just, and Free America Biosecurity: A Comprehensive Action Plan Center for American Progress Biosecurity A Comprehensive Action Plan Andrew J. Grotto and Jonathan B. Tucker June 2006 After Guantanamo: A Special Tribunal for International Terrorist Suspects 1 Biosecurity: A Comprehensive Action Plan Center for American Progress Table of Contents EXECUTIVE SUMMARY i BIOLOGICAL THREATS FACING THE UNITED STATES 1 PREVENTING BIO-CATASTROPHES: THE NEED FOR A GLOBAL APPROACH 9 PREVENTING THE MISUSE OF THE LIFE SCIENCES 9 RECOMMENDATIONS STRENGTHENING BIOLOGICAL DISARMAMENT MEASURES 14 RECOMMENDATIONS 8 CONTAINING DISEASE OUTBREAKS: AN INTEGRATED PUBLIC HEALTH STRATEGY 21 TIMELY DETECTION OF OUTBREAKS 22 RECOMMENDATIONS 0 RAPID CONTAINMENT OF OUTBREAKS 32 RECOMMENDATIONS 5 DEFENDING AGAINST BIOLOGICAL THREATS: AN INTEGRATED RESEARCH STRATEGY 37 REFORMING THE DRUG DEVELOPMENT PROCESS 38 RECOMMENDATIONS 41 RATIONALIZING BIODEFENSE SPENDING 42 RECOMMENDATIONS 44 GLOSSARY 47 Biosecurity: A Comprehensive Action Plan ACKNOWLEDGMENTS The authors are deeply grateful to the following individuals for their valuable comments and criticisms on earlier drafts of this report: Bob Boorstin, Joseph Cirincione, P. J. Crowley, Richard Ebright, Gerald L. Epstein, Trevor Findlay, Brian Finlay, Elisa D. Harris, David Heyman, Ajey Lele, Dan Matro, Caitriona McLeish, Jonathan Moreno, Peter Ogden, Alan Pearson, Michael Schiffer, Laura Segal, and Bradley Smith. 4 Center for American Progress Executive Summary iological weapons and infectious diseases share several fundamental characteristics that the United States can leverage to counter both Bof these threats more effectively. Both a bioweapons attack and a natural pandemic, such as avian flu, can be detected in similar ways, and the effectiveness of any response to an outbreak of infectious disease, whether natural or caused deliberately by terrorists, hinges on the strength of the U.S. -

A Review of the Efficacy of FDA-Approved B. Anthracis Anti
toxins Review A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models Zoe Couse 1, Xizhong Cui 1, Yan Li 1, Mahtab Moayeri 2, Stephen Leppla 2 and Peter Q. Eichacker 1,* 1 Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (Z.C.); [email protected] (X.C.); [email protected] (Y.L.) 2 National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.M.); [email protected] (S.L.) * Correspondence: [email protected] Abstract: Anti-toxin agents for severe B. anthracis infection will only be effective if they add to the benefit of the two mainstays of septic shock management, antibiotic therapy and titrated hemody- namic support. Both of these standard therapies could negate benefits related to anti-toxin treatment. At present, three anthrax anti-toxin antibody preparations have received US Food and Drug Adminis- tration (FDA) approval: Raxibacumab, Anthrax Immune Globulin Intravenous (AIGIV) and ETI-204. Each agent is directed at the protective antigen component of lethal and edema toxin. All three agents were compared to placebo in antibiotic-treated animal models of live B. anthracis infection, and Raxibacumab and AIGIV were compared to placebo when combined with standard hemodynamic support in a 96 h canine model of anthrax toxin-associated shock. However, only AIG has actually Citation: Couse, Z.; Cui, X.; Li, Y.; been administered to a group of infected patients, and this experience was not controlled and offers Moayeri, M.; Leppla, S.; Eichacker, P.Q. -

The Project Bioshield Act: Issues for the 112Th Congress
The Project BioShield Act: Issues for the 112th Congress Frank Gottron Specialist in Science and Technology Policy March 13, 2012 Congressional Research Service 7-5700 www.crs.gov R42349 CRS Report for Congress Prepared for Members and Committees of Congress The Project BioShield Act: Issues for the 112th Congress Summary In 2004, Congress passed the Project BioShield Act (P.L. 108-276) to provide the federal government with new authorities related to the development, procurement, and use of medical countermeasures against chemical, biological, radiological, and nuclear (CBRN) terrorism agents. As the expiration of some of these authorities approaches, Congress is considering whether these authorities have sufficiently contributed to national preparedness to merit extension. The Project BioShield Act provides three main authorities: (1) guaranteeing a federal market for new CBRN medical countermeasures, (2) permitting emergency use of countermeasures that are either unapproved or have not been approved for the intended emergency use, and (3) relaxing regulatory requirements for some CBRN terrorism-related spending. The Department of Health and Human Services (HHS) has used each of these authorities. The HHS obligated approximately $2.5 billion to guarantee a government market for countermeasures against anthrax, botulism, radiation, and smallpox. The HHS allowed the emergency use of several unapproved products, including during the 2009 H1N1 influenza pandemic. The HHS used expedited review authorities to approve contracts and grants related to CBRN countermeasure research and development. The Department of Homeland Security (DHS) Appropriations Act, 2004 (P.L. 108-90) advance- appropriated $5.593 billion to acquire CBRN countermeasures through Project BioShield for FY2004-FY2013. Through FY2012, subsequent Congresses have removed $1.876 billion from this account through rescissions and transfers, more than one-third of the advance appropriation. -

Project Bioshield Act Fact Sheet
ASTHO LEGAL PREPAREDNESS SERIES EMERGENCY USE AUTHORIZATION TOOLKIT Project BioShield Act Fact Sheet Overview With the anthrax and other terrorist attacks of 2001, the federal government determined that it would need new medical countermeasures, such as diagnostic tests, drugs, and vaccines, to respond to an attack using chemical, biological, radiological, or nuclear (CBRN) agents.1 The lack of a significant commercial market for CBRN countermeasures was identified as a reason for the dearth of such countermeasures.1 Since diseases and conditions caused by CBRN agents occur infrequently, the private sector did not have an economic incentive to invest the millions of dollars necessary to research and develop such measures.1 The Project BioShield Act of 20042 was enacted to accelerate the research, development, purchase, and availability of effective medical products against CBRN agents.3 Project BioShield provided the government with the authority and funding to develop, acquire, stockpile, and distribute the medical products needed to protect the United States against weapons of mass destruction.3 Note: As of March 2012, Congress is in the process of reauthorizing the Pandemic and All-Hazards Preparedness Act (PAHPA), which may impact some provisions of the Project BioShield Act. Please see ASTHO EUA Current Issues Winter 2012 for more information about reauthorization and its potential impact on EUAs and related issues. What the Law Does The Project BioShield Act authorizes expedited procurement, streamlined personnel appointments, expedited peer review, biomedical countermeasures procurement, emergency use of medical countermeasures, and other biodefense activities. Liability Protections The Project BioShield Act does not contain any explicit immunity or other liability protections. -

Failures of Project Bioshield & Congressional Attempts to Remedy It
DePaul Journal of Health Care Law Volume 10 Issue 3 Spring 2007 Article 8 October 2015 Failures of Project BioShield & Congressional Attempts to Remedy It Megan O'Reilly Follow this and additional works at: https://via.library.depaul.edu/jhcl Recommended Citation Megan O'Reilly, Failures of Project BioShield & Congressional Attempts to Remedy It, 10 DePaul J. Health Care L. 503 (2007) Available at: https://via.library.depaul.edu/jhcl/vol10/iss3/8 This Case Briefs is brought to you for free and open access by the College of Law at Via Sapientiae. It has been accepted for inclusion in DePaul Journal of Health Care Law by an authorized editor of Via Sapientiae. For more information, please contact [email protected]. CASE BRIEF: THE FAILURES OF PROJECT BIOSHIELD & CONGRESSIONAL ATTEMPTS TO REMEDY IT Megan O'Reilly I. THE THREAT OF A BIOLOGICAL TERROR ATTACK In response to the 2001 terrorist attacks, the United States government began a crash program to develop drugs, vaccine and diagnostic tests to protect the nation from biological terrorism.' As part of this effort, President Bush signed Project BioShield into law on July 21, 2004. The legislation was intended to facilitate a faster process to research, develop, purchase and make bioterrorism countermeasures available to combat bioterrorist threats. 2 However, Project BioShield is not drawing the interest it aimed for. The legislation has faced significant criticism because the law has failed to adequately provide consistent and well-coordinated financing, and sufficient, ensured markets, as well as any liability protection for vaccine developers. 3 The threat of a biological attack is very real.4 To ensure that Project BioShield assists in protecting the nation from future bioterrorist attacks, it is necessary to evaluate the successes and setbacks the initiative has faced. -
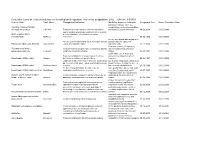
Designated Indication Cumulative List of All Products That Have Received
Cumulative List of all Products that have received Orphan Designation: Total active designations: 2002 Effecive: 5/5/2009 Generic Name Trade Name Designated Indication treatmentMarketing and/or Approved modification Indication of the Designated Date Market Exculsivity Date following conditions, which are Vaccinia Immune Globulin complications resulting from smallpox (Human) Intravenous CNJ-016 ForTreatment the control of complications and prevention of vacciniaof hemorrhagic vaccination episodes vaccination: Eczema vaccinatum 18.06.2004 01.05.9999 and for surgical prophylaxis in patients with hemophilia Antihemophilic factor A (congenital factor VIII deficiency or classic (recombinant) ReFacto hemophilia). 08.02.1996 01.01.9999 For use as a thyroid blocking agent in For use as a thyroid blocking agent in pediatric patients pediatric patients exposed to Potassium Iodide Oral Solution ThyroShield exposed to radiactive iodine radiactive iodine 17.11.2004 01.01.9999 Treatment (rescue) of respiratory Pulmonary surfactant For the treatment and prevention of respiratory distress distress syndrome in premature replacement, porcine Curosurf syndrome in premature infants. Longinfants. term treatment of children with 02.08.1993 01.01.9999 growth failure due to inadequate Treatment of idiopathic or organic growth hormone secretion of endogenous growth Somatropin (rDNA origin) Saizen deficiency in children with growth failure. Long-termhormone. treatment of growth failure 06.03.1987 01.01.9999 Long-term treatment of children who have growth failure due to a lack of adequate endogenous due to a lack of adequate endogenous growth hormone growth hormone secetion for once- or Somatropin (rDNA origin) Nutropin Depot secretion. twice-a-month administration. 28.10.1999 01.01.9999 Treatment of growth failure in children due to have growth failure due to inadequate Somatropin (rDNA origin) injection Norditropin inadequate growth hormone secretion. -

Public Law 108–276 108Th Congress An
PUBLIC LAW 108–276—JULY 21, 2004 118 STAT. 835 Public Law 108–276 108th Congress An Act To amend the Public Health Service Act to provide protections and countermeasures against chemical, radiological, or nuclear agents that may be used in a terrorist attack against the United States by giving the National Institutes of Health July 21, 2004 contracting flexibility, infrastructure improvements, and expediting the scientific [S. 15] peer review process, and streamlining the Food and Drug Administration approval process of countermeasures. Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, Project BioShield Act of 2004. SECTION 1. SHORT TITLE. 42 USC 201 note. This Act may be cited as the ‘‘Project BioShield Act of 2004’’. SEC. 2. BIOMEDICAL COUNTERMEASURE RESEARCH AND DEVELOP- MENT—AUTHORITIES. (a) IN GENERAL.—Part B of title III of the Public Health Service Act (42 U.S.C. 243 et seq.) is amended by inserting after section 319F the following section: ‘‘SEC. 319F–1. AUTHORITY FOR USE OF CERTAIN PROCEDURES 42 USC 247d–6a. REGARDING QUALIFIED COUNTERMEASURE RESEARCH AND DEVELOPMENT ACTIVITIES. ‘‘(a) IN GENERAL.— ‘‘(1) AUTHORITY.—In conducting and supporting research and development activities regarding countermeasures under section 319F(h), the Secretary may conduct and support such activities in accordance with this section and, in consultation with the Director of the National Institutes of Health, as part of the program under section 446, if the activities concern qualified countermeasures. ‘‘(2) QUALIFIED COUNTERMEASURE.—For purposes of this section, the term ‘qualified countermeasure’ means a drug (as that term is defined by section 201(g)(1) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C.