Monoclonal Antibody Therapies Against Anthrax
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ANTHRASIL™ Safely and Effectively
53 Weight-based Pediatric Dose Body Weight Vials per Dosea Body Weight Vials per Dose (kg) (kg) 1 HIGHLIGHTS OF PRESCRIBING INFORMATION <5 1 25 to <35 4 <10 1 35 to <50 5 10 to <18 2 50 to <60 6 These highlights do not include all the information needed to use 2 18 to <25 3 ≥60 7 ANTHRASIL™ safely and effectively. See full prescribing information for 3 aSelect initial dose based on clinical severity. Dose may be doubled for severe ANTHRASIL. 4 cases in patients >5 kg. 5 ANTHRASIL [Anthrax Immune Globulin Intravenous (Human)], sterile 54 6 Administer ANTHRASIL by slow intravenous infusion using an infusion solution for infusion 55 7 pump (maximum 2 mL per minute). 56 8 Initial U.S. Approval: March 24, 2015 57 9 ---------------------DOSAGE FORMS AND STRENGTHS---------------------- 10 58 59 Each single-use vial contains a minimum potency of ≥60 units by Toxin 11 WARNING: INTERACTIONS WITH GLUCOSE MONITORING 60 Neutralization Assay (TNA) (3). 12 SYSTEMS AND THROMBOSIS 61 13 See full prescribing information for complete boxed warning. 62 -------------------------------CONTRAINDICATIONS------------------------------ 14 • Maltose in immune globulin products, including ANTHRASIL, may give 63 • History of anaphylactic or severe systemic reaction to human immune 15 falsely high blood glucose levels with some blood point-of-care glucose 64 globulins (4) 16 testing systems (for example those based on the GDH-PQQ or glucose-dye- 65 • IgA deficiency with antibodies against IgA and a history of IgA 17 oxidoreductase methods) resulting in inappropriate administration of insulin 66 hypersensitivity (4) 18 and life-threatening hypoglycemia. To avoid interference by maltose 67 19 contained in ANTHRASIL, perform blood glucose measurements in patients 68 -----------------------WARNINGS AND PRECAUTIONS------------------------ 20 receiving ANTHRASIL with a glucose-specific method (monitor and test 21 strips). -

These Highlights Do Not Include All the Information Needed to Use ANTHIM Safely and Effectively. See Full Prescribing Information for ANTHIM
ANTHIM - obiltoxaximab solution Elusys Therapeutics, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ANTHIM safely and effectively. See full prescribing information for ANTHIM. ANTHIM® (obiltoxaximab) injection, for intravenous use Initial U.S. Approval: 2016 WARNING: HYPERSENSITIVITY and ANAPHYLAXIS See full prescribing information for complete boxed warning. Hypersensitivity reactions, including anaphylaxis, have been reported during ANTHIM infusion (5.1) ANTHIM should be administered in monitored settings by personnel trained and equipped to manage anaphylaxis (1.2, 2.4, 5.1) Stop ANTHIM infusion immediately and treat appropriately if hypersensitivity or anaphylaxis occurs (2.4, 5.1) INDICATIONS AND USAGE ANTHIM® is a monoclonal antibody directed against the protective antigen of Bacillus anthracis. It is indicated in adult and pediatric patients for treatment of inhalational anthrax due to B. anthracis in combination with appropriate antibacterial drugs and, for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate. (1.1) Limitations of Use ANTHIM should only be used for prophylaxis when its benefit for prevention of inhalational anthrax outweighs the risk of hypersensitivity and anaphylaxis. (1.2, 5.1) The effectiveness of ANTHIM is based solely on efficacy studies in animal models of inhalational anthrax. (1.2, 14) There have been no studies of the safety or pharmacokinetics (PK) of ANTHIM in the pediatric population. Dosing in pediatric patients was derived using a population PK approach. (1.2, 8.4) ANTHIM does not have direct antibacterial activity. ANTHIM should be used in combination with appropriate antibacterial drugs. (1.2) ANTHIM is not expected to cross the blood-brain barrier and does not prevent or treat meningitis. -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery. -

The Effect of Raxibacumab on the Immunogenicity of Anthrax Vaccine Adsorbed: a Phase
The effect of raxibacumab on the immunogenicity of anthrax vaccine adsorbed: a Phase IV, randomised, open-label, parallel-group, non-inferiority study Nancy Skoura, PhD1, Jie Wang-Jairaj, MD2, Oscar Della Pasqua MD2, Vijayalakshmi Chandrasekaran, MS1, Julia Billiard, PhD1, Anne Yeakey, MD3, William Smith, MD4, Helen Steel, MD2, Lionel K Tan, FRCP2 1GlaxoSmithKline, Inc. Collegeville, PA, USA 2GlaxoSmithKline. Stockley Park West, Middlesex, UK 3GlaxoSmithKline, Inc. Rockville, MD, USA 4AMR, at University of TN Medical Center, Knoxville, TN; New Orleans Center for Clinical Research (NOCCR), USA Author for Correspondence: Dr Lionel K Tan GlaxoSmithKline Stockley Park West 1–3 Ironbridge Road Uxbridge Middlesex UB11 1BT UK Email: [email protected] Tel: +44 (0)7341 079 683 1 Abstract: 340/350 Body text: 4321/4500 words including Research in Context Table/Figures: 2/4 References: 30 2 Abstract Background Raxibacumab is a monoclonal antibody (Ab) which binds protective antigen (PA) of Bacillus anthracis and is approved for treatment and post-exposure prophylaxis (PEP) of inhalational anthrax. Anthrax vaccine adsorbed (AVA), for anthrax prophylaxis, consists primarily of adsorbed PA. This post-approval study evaluated the effect of raxibacumab on immunogenicity of AVA. Methods In this open-label, parallel-group, non-inferiority study in three centres in the USA, healthy volunteers (aged 18–65 years) with no evidence of PA pre-exposure were randomised 1:1 to receive either subcutaneous 0·5 mL AVA on Days 1, 15, and 29 or raxibacumab intravenous infusion (40 mg/kg) immediately before AVA on Day 1, followed by AVA only on Days 15 and 29. -

125509Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125509Orig1s000 ADMINISTRATIVE and CORRESPONDENCE DOCUMENTS ACTION PACKAGE CHECKLIST APPLICATION INFORMATION1 If NDA, Efficacy Supplement Type: BLA #125509 BLA Supplement # (an action package is not required for SE8 or SE9 supplements) Proprietary Name: Anthim Applicant: Elusys Therapeutics, Inc. Established/Proper Name: obiltoxaximab Agent for Applicant (if applicable): Dosage Form: injection RPM: Jane A. Dean, RN, MSN Division: Division of Anti-Infective Products For ALL 505(b)(2) applications, two months prior to EVERY action: NDA Application Type: 505(b)(1) 505(b)(2) Efficacy Supplement: 505(b)(1) 505(b)(2) Review the information in the 505(b)(2) Assessment and submit the draft2 to CDER OND IO for clearance. BLA Application Type: 351(k) 351(a) Check Orange Book for newly listed patents and/or Efficacy Supplement: 351(k) 351(a) exclusivity (including pediatric exclusivity) No changes New patent/exclusivity (notify CDER OND IO) Date of check: Note: If pediatric exclusivity has been granted or the pediatric information in the labeling of the listed drug changed, determine whether pediatric information needs to be added to or deleted from the labeling of this drug. Actions Proposed action AP TA CR User Fee Goal Date is 3/20/16 Previous actions (specify type and date for each action taken) None If accelerated approval or approval based on efficacy studies in animals, were promotional materials received? Note: Promotional materials to be used within 120 days after approval must have been Received submitted (for exceptions, see http://www fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guida nces/ucm069965.pdf). -

Ride'n Drive on Government Waste
NEWS Table 1 Anthrax countermeasures in development Company Product Stage of development BARDA contract Human Genome Sciences ABthrax (raxibacumab), a human mAb against anthrax protective Biologic license $151 million antigen (PA) application Emergent BioSolutions AV-7909, a combination of BioThrax (aluminum-adsorbed cell- Phase 2 $447.6 million free filtrates of unencapsulated Bacillus anthracis) and Coley Pharmaceutical’s VaxImmune (an unmethylated CpG-motif oligonucleotide that acts as an agonist of Toll-like receptor 9) Anthrax immune globulin (AIGIV), polyclonal antibodies raised Phase 1/2 $13 million against BioThrax Elusys Therapeutics Anthim (ETI-204), a humanized mAb against PA Phase 1 Up to $143 million PharmAthene SparVax, an injectable rPA absorbed on to hydrogel Phase 2 $3.9 million NIAID DynPort Vaccine Anthrax vaccine, an injectable rPA vaccine Phase 1 NA Medarex, a subsidiary of Valortim (MDX-1303), a fully human mAb against PA Phase 1 $1 million from the US Department Bristol-Myers Squibb of Defense (DoD) payable to partner PHarmAthene Advanced Life Sciences Restanza, a once-daily oral ketolide cethromycin that inhibits Preclinical $3.8 million from DoD B. anthracis protein synthesis Sources: Sagient Research, BiomedTracker and BARDA. NA, Not available. Even with government support, PharmAthene For the US government, anthrax treatment procurement contract with the Centers for and other companies working under biodefense remains a critical element in its biodefense Disease Control and Prevention of Atlanta, contracts face the stark reality of drug develop- strategies. Emergent Solutions currently Georgia, to manufacture and deliver 14.5 ment: 30% of all candidates that reach phase manufactures the only approved vaccine for million doses of BioThrax for the strategic 3 clinical trials within the eight-year BARDA anthrax based on protective antigen (PA). -

A Review of the Efficacy of FDA-Approved B. Anthracis Anti
toxins Review A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models Zoe Couse 1, Xizhong Cui 1, Yan Li 1, Mahtab Moayeri 2, Stephen Leppla 2 and Peter Q. Eichacker 1,* 1 Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (Z.C.); [email protected] (X.C.); [email protected] (Y.L.) 2 National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.M.); [email protected] (S.L.) * Correspondence: [email protected] Abstract: Anti-toxin agents for severe B. anthracis infection will only be effective if they add to the benefit of the two mainstays of septic shock management, antibiotic therapy and titrated hemody- namic support. Both of these standard therapies could negate benefits related to anti-toxin treatment. At present, three anthrax anti-toxin antibody preparations have received US Food and Drug Adminis- tration (FDA) approval: Raxibacumab, Anthrax Immune Globulin Intravenous (AIGIV) and ETI-204. Each agent is directed at the protective antigen component of lethal and edema toxin. All three agents were compared to placebo in antibiotic-treated animal models of live B. anthracis infection, and Raxibacumab and AIGIV were compared to placebo when combined with standard hemodynamic support in a 96 h canine model of anthrax toxin-associated shock. However, only AIG has actually Citation: Couse, Z.; Cui, X.; Li, Y.; been administered to a group of infected patients, and this experience was not controlled and offers Moayeri, M.; Leppla, S.; Eichacker, P.Q. -

The Project Bioshield Act: Issues for the 112Th Congress
The Project BioShield Act: Issues for the 112th Congress Frank Gottron Specialist in Science and Technology Policy March 13, 2012 Congressional Research Service 7-5700 www.crs.gov R42349 CRS Report for Congress Prepared for Members and Committees of Congress The Project BioShield Act: Issues for the 112th Congress Summary In 2004, Congress passed the Project BioShield Act (P.L. 108-276) to provide the federal government with new authorities related to the development, procurement, and use of medical countermeasures against chemical, biological, radiological, and nuclear (CBRN) terrorism agents. As the expiration of some of these authorities approaches, Congress is considering whether these authorities have sufficiently contributed to national preparedness to merit extension. The Project BioShield Act provides three main authorities: (1) guaranteeing a federal market for new CBRN medical countermeasures, (2) permitting emergency use of countermeasures that are either unapproved or have not been approved for the intended emergency use, and (3) relaxing regulatory requirements for some CBRN terrorism-related spending. The Department of Health and Human Services (HHS) has used each of these authorities. The HHS obligated approximately $2.5 billion to guarantee a government market for countermeasures against anthrax, botulism, radiation, and smallpox. The HHS allowed the emergency use of several unapproved products, including during the 2009 H1N1 influenza pandemic. The HHS used expedited review authorities to approve contracts and grants related to CBRN countermeasure research and development. The Department of Homeland Security (DHS) Appropriations Act, 2004 (P.L. 108-90) advance- appropriated $5.593 billion to acquire CBRN countermeasures through Project BioShield for FY2004-FY2013. Through FY2012, subsequent Congresses have removed $1.876 billion from this account through rescissions and transfers, more than one-third of the advance appropriation. -

Statistical Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125509Orig1s000 STATISTICAL REVIEW(S) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Office of Translational Sciences Office of Biostatistics S TATISTICAL R EVIEW AND E VA LU ATI O N ANIMAL EFFICACY STUDIES NDA/BLA #: BLA 125509 Drug Name: Anthim®(obiltoxaximab) 16 mg/kg IV Indication(s): Treatment and Prophylaxis of Inhalational Anthrax due to Bacillus anthracis Applicant: Elusys Therapeutics, Inc. Date(s): Submission date: 3/20/2015. PDUFA due date: 3/18/2016 Review Priority: Standard Biometrics Division: Division of Biometrics IV Statistical Reviewer: Xianbin Li, PhD Concurring Reviewers: Karen Higgins, ScD Daphne Lin, PhD Medical Division: Division of Anti-Infective Products (DAIP) Clinical Team: Elizabeth O'Shaughnessy, MD, Medical Reviewer John Alexander, MD, Medical Team Leader Sumathi Nambiar, MD, Medical Director Project Manager: Jane Dean, RN MSN Keywords: Animal efficacy studies Reference ID: 3859664 Table of Contents 1 EXECUTIVE SUMMARY .................................................................................................. 11 2 INTRODUCTION ................................................................................................................ 12 2.1 OVERVIEW ........................................................................................................................ 12 2.2 DATA SOURCES ............................................................................................................... -
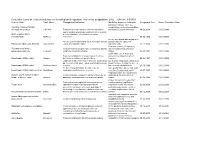
Designated Indication Cumulative List of All Products That Have Received
Cumulative List of all Products that have received Orphan Designation: Total active designations: 2002 Effecive: 5/5/2009 Generic Name Trade Name Designated Indication treatmentMarketing and/or Approved modification Indication of the Designated Date Market Exculsivity Date following conditions, which are Vaccinia Immune Globulin complications resulting from smallpox (Human) Intravenous CNJ-016 ForTreatment the control of complications and prevention of vacciniaof hemorrhagic vaccination episodes vaccination: Eczema vaccinatum 18.06.2004 01.05.9999 and for surgical prophylaxis in patients with hemophilia Antihemophilic factor A (congenital factor VIII deficiency or classic (recombinant) ReFacto hemophilia). 08.02.1996 01.01.9999 For use as a thyroid blocking agent in For use as a thyroid blocking agent in pediatric patients pediatric patients exposed to Potassium Iodide Oral Solution ThyroShield exposed to radiactive iodine radiactive iodine 17.11.2004 01.01.9999 Treatment (rescue) of respiratory Pulmonary surfactant For the treatment and prevention of respiratory distress distress syndrome in premature replacement, porcine Curosurf syndrome in premature infants. Longinfants. term treatment of children with 02.08.1993 01.01.9999 growth failure due to inadequate Treatment of idiopathic or organic growth hormone secretion of endogenous growth Somatropin (rDNA origin) Saizen deficiency in children with growth failure. Long-termhormone. treatment of growth failure 06.03.1987 01.01.9999 Long-term treatment of children who have growth failure due to a lack of adequate endogenous due to a lack of adequate endogenous growth hormone growth hormone secetion for once- or Somatropin (rDNA origin) Nutropin Depot secretion. twice-a-month administration. 28.10.1999 01.01.9999 Treatment of growth failure in children due to have growth failure due to inadequate Somatropin (rDNA origin) injection Norditropin inadequate growth hormone secretion. -

PRESCRIBING INFORMATION • Pre-Medicate with Diphenhydramine
----------------------- DOSAGE AND ADMINISTRATION ----------------------- HIGHLIGHTS OF PRESCRIBING INFORMATION • Pre-medicate with diphenhydramine. (2.1, 5.1) These highlights do not include all the information needed to use • Recommended Dosage of ANTHIM: ANTHIM safely and effectively. See full prescribing information for o Adult Patients: 16 mg/kg. (2.1) ANTHIM. o Pediatric Patients: (2.2) ➢ Greater than 40 kg: 16 mg/kg ANTHIM® (obiltoxaximab) injection, for intravenous use ➢ Greater than 15 kg to 40 kg: 24 mg/kg Initial U.S. Approval: 2016 ➢ Less than or equal to 15 kg: 32 mg/kg • Dilute the injection in 0.9% Sodium Chloride Injection, USP, before WARNING: HYPERSENSITIVITY and ANAPHYLAXIS administering as an intravenous (IV) infusion over 1 hour and 30 minutes. See full prescribing information for complete boxed warning. (2.3) • Administer ANTHIM in a monitored setting equipped to manage • Hypersensitivity reactions, including anaphylaxis, have been anaphylaxis. (2.4, 5.1) reported during ANTHIM infusion (5.1) • See Full Prescribing Information for instructions on preparation, dilution • ANTHIM should be administered in monitored settings by and administration of ANTHIM injection. (2.3, 2.4) personnel trained and equipped to manage anaphylaxis (1.2, 2.4, 5.1) --------------------- DOSAGE FORMS AND STRENGTHS --------------------- • Stop ANTHIM infusion immediately and treat appropriately if Injection: 600 mg/6 mL (100 mg/mL) solution in single-dose vial. (3) hypersensitivity or anaphylaxis occurs (2.4, 5.1) ------------------------------ CONTRAINDICATIONS ------------------------------ None (4) --------------------------- INDICATIONS AND USAGE ---------------------------- ANTHIM® is a monoclonal antibody directed against the protective antigen of ----------------------- WARNINGS AND PRECAUTIONS ----------------------- Bacillus anthracis. It is indicated in adult and pediatric patients for treatment Hypersensitivity reactions, including anaphylaxis (Boxed Warning, 1.2, 2.1, of inhalational anthrax due to B. -

Ibm Micromedex® Carenotes Titles by Category
IBM MICROMEDEX® CARENOTES TITLES BY CATEGORY DECEMBER 2019 © Copyright IBM Corporation 2019 All company and product names mentioned are used for identification purposes only and may be trademarks of their respective owners. Table of Contents IBM Micromedex® CareNotes Titles by Category Allergy and Immunology ..................................................................................................................2 Ambulatory.......................................................................................................................................3 Bioterrorism ...................................................................................................................................18 Cardiology......................................................................................................................................18 Critical Care ...................................................................................................................................20 Dental Health .................................................................................................................................22 Dermatology ..................................................................................................................................23 Dietetics .........................................................................................................................................24 Endocrinology & Metabolic Disease ..............................................................................................26