<I>Winteraceae</I>
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Phytophthora Ramorum Sudden Oak Death Pathogen
NAME OF SPECIES: Phytophthora ramorum Sudden Oak Death pathogen Synonyms: Common Name: Sudden Oak Death pathogen A. CURRENT STATUS AND DISTRIBUTION I. In Wisconsin? 1. YES NO X 2. Abundance: 3. Geographic Range: 4. Habitat Invaded: 5. Historical Status and Rate of Spread in Wisconsin: 6. Proportion of potential range occupied: II. Invasive in Similar Climate YES NO X Zones United States: In 14 coastal California Counties and in Curry County, Oregon. In nursery in Washington. Canada: Nursery in British Columbia. Europe: Germany, the Netherlands, the United Kingdom, Poland, Spain, France, Belgium, and Sweden. III. Invasive in Similar Habitat YES X NO Types IV. Habitat Affected 1. Habitat affected: this disease thrives in cool, wet climates including areas in coastal California within the fog belt or in low- lying forested areas along stream beds and other bodies of water. Oaks associated with understory species that are susceptible to foliar infections are at higher risk of becoming infected. 2. Host plants: Forty-five hosts are regulated for this disease. These hosts have been found naturally infected by P. ramorum and have had Koch’s postulates completed, reviewed and accepted. Approximately fifty-nine species are associated with Phytophthora ramorum. These species are found naturally infected; P. ramorum has been cultured or detected with PCR but Koch’s postulates have not been completed or documented and reviewed. Northern red oak (Quercus rubra) is considered an associated host. See end of document for complete list of plant hosts. National Risk Model and Map shows susceptible forest types in the mid-Atlantic region of the United States. -

Supporting Information
Supporting Information Bachelier and Friedman 10.1073/pnas.1104697108 Table S1. Initiation of more than one female gametophyte per ovule in basal angiosperms Taxon Multiple female gametophytes References Amborellaceae No 1–4 Hydatellaceae Variable 5 and 6 Nymphaeaceae Variable 7–9 Cabombaceae Variable 8 and 10 Austrobaileyaceae No 11 Illiciaceae (incl. Schisandraceae) Variable 12–15 Trimeniaceae Variable 16–18 and this study Chloranthaceae Rare 4 and 18–22 Laurales Variable 18 and 23–27 Magnoliales No 27–31 Canellales Rare 30 and 32–37 Piperales Rare 38–44 Ceratophyllaceae Variable 45 Character states: No means that initiation of more than one female gametophyte per ovule has not been reported as yet, rare means that initiation of more than one female gametophyte per ovule has only been reported one time, and variable means that initiation of more than one female gametophyte per ovule has been reported to occur more than one time in some but not all studies. 1. Friedman WE, Ryerson KC (2009) Reconstructing the ancestral female gametophyte of angiosperms: Insights from Amborella and other ancient lineages of flowering plants. Am J Bot 96:129–143. 2. Friedman WE (2006) Embryological evidence for developmental lability during early angiosperm evolution. Nature 441:337–340. 3. Tobe H, Jaffré T, Raven PH (2000) Embryology of Amborella (Amborellaceae): Descriptions and polarity of character states. J Plant Res 113:271–280. 4. Yamada T, Tobe H, Imaichi R, Kato M (2001) Developmental morphology of the ovules of Amborella trichopoda (Amborellaceae) and Chloranthus serratus (Chloranthaceae). Bot J Linn Soc 137:277–290. 5. Rudall PJ, et al. -

Tasmannia Lanceolata
ASPECTS OF LEAF AND EXTRACT PRODUCTION from Tasmannia lanceolata by Chris Read, B. Agr.Sc. Tas. Submitted in fulfillment of the requirements for the Degree of Doctor of Philosophy University of Tasmania, Hobart December 1995 ' s~, ... ~~ \ ·'(11 a_C\14 \t\J. \I ' This thesis contains no material which has been accepted for the award of any other degree or diploma in any University, and to the best of my knowledge, contains no copy or paraphrase of material previously written or published by any other person except where due reference is given in the text. University of Tasmania HOBART March 1996 This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968 University of Tasmania HOBART March 1996 Abstract This thesis examines several aspects of the preparation, extraction and analysis of solvent soluble compounds from leaf material of Tasmannia lanceolata and reports a preliminary survey of extracts of some members of the natural population of the species in Tasmania. A major constituent of these extracts, polygodial, was shown to be stored within specialised idioblastic structures scattered throughout the mesophyll, and characterised by distinctive size and shape, and a thickened wall. The contents of these cells were sampled directly, analysed and compared with the composition of extracts derived from ground, dry whole leaf. This result was supported by spectroscopic analysis of undisturbed oil cells in whole leaf tissue. In a two year field trial, the progressive accumulation of a number of leaf extract constituents (linalool, cubebene, caryophyllene, germacrene D, bicyclogermacrene, cadina-1,4 - diene, aristolone and polygodial) during the growth flush was followed by a slow decline during the subsequent dormant season. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

And According to Bailey & (1943)
BLUMEA 24 (1978) 521—525 The Winteraceae pf the Old World. III. Notes on the ovary of Takhtajania W. Vink Rijksherbarium, Leiden, Netherlands INTRODUCTION Very recently Baranova & Leroy (Leroy, 1978) published a new genus Takhta- jania to accomodate the aberrant Bubbia perrieri Capuron. The outstanding characters of this genus are the anomocytic stomatal apparatus (Baranova, 1972; Bongers, 1973) and the unilocular bicarpellate ovary (Leroy, 1977, 1978). work the Winteraceae, also studied the of Bub- During my on I single specimen bia perrieri in existence. The late Capuron told me that he had tried to collect additional specimens but that he had not succeeded in doing so; according to him the type locality was completely deforested. In view of the scarcity of the material it was considered relevant to publish some additional notes without delay. MATERIAL AND METHODS I studied three ovaries of the type specimen (Perrier de la Bdthie 10158). One was cut longitudinally, slightly outside the plane of symmetry; another was cut and its half These drawn transversally upper again longitudinally. parts were (figs. 2 and 3) and then cleared according to Bailey & Nast (1943) (figs 5 and third boiled and then sectioned 6). The ovary was serially (fig. 4). OBSERVATIONS One of the ovaries was taken from a flowerbud of which the closed petals were 57 mm high. The diagram of this flowerbud is drawn in fig. 1. The 2 slightly ruptured calyx showed three putative original apices ± alternating with the three bracteoles at the base of the pedicel. The petals are all free. The ovary is its and the flattened, has a longitudinal groove on narrow sides, highest sta- mens are inserted adjoining its broadest sides. -

<I>Winteraceae</I>
Blumea 59, 2014: 155–162 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE http://dx.doi.org/10.3767/000651914X686022 The Winteraceae of the Old World. VII. Zygogynum in the Solomon Islands (incl. Bougainville) W. Vink1 Key words Abstract A review of the character distribution in the Zygogynum representatives from the Solomon Islands (and Bougainville) shows a rather wide variability in ranges of numbers and sizes, often with distinct differences between Solomon Islands islands or altitudinal ranges. However, the morphology is rather uniform. The four species recognised by A.C. Smith Winteraceae had to be synonymised, but a new taxon deserved recognition. The name Bubbia is explained. Zygogynum Published on 19 December 2014 INTRODUCTION CHARACTERS AND THEIR VARIABILITY The first Zygogynum species described from the Solomon Is- Leaves lands is Bubbia haplopus B.L.Burtt (1936). In his monumental Except for size, the leaves are very uniform with the blade works on the Papuasian plants, Smith (1942) transferred this chartaceous, the midrib impressed above and prominent below. species from Bubbia to the other Van Tieghem genus, Belliolum, On the lower side of the blade the grey to white alveolar mate- and described three more species from this area. The materials rial covers only the stomata or unites these into small strings available to Smith were, however, very incomplete, and several or clusters; the stomata are also present over the secondary characters could not be established; next to leaf size, minor nerves, or less dense to lacking over the thickest parts of the differences had to be used to define these taxa. -

Notification of Access Arrangement for MP 41279, Mt Te Kuha
Attachment C Draft Terrestrial Ecology Report 106 VEGETATION AND FAUNA OF THE PROPOSED TE KUHA MINE SITE Prepared for Te Kuha Limited Partnership October 2013 EXECUTIVE SUMMARY The Te Kuha mining permit is located predominantly within the Westport Water Conservation Reserve (1,825 ha), which is a local purpose reserve administered by the Buller District Council. The coal deposit is situated outside the water catchment within an area of approximately 490 ha of Brunner Coal Measures vegetation approximately 5 km southwest of Mt Rochfort. Access would be required across conservation land to reach the coal resource. The Te Kuha site was recommended as an area for protection by the Protected Natural Areas Programme surveys in the 1990s on the basis that in the event it was removed from the local purpose reserve for any reason, addition to the public conservation estate would increase the level of protection of coal measures habitats which, although found elsewhere (principally in the Mt Rochfort Conservation Area), were considered inadequately protected overall. The proposal to create an access road and an opencast mine at the site would affect twelve different vegetation types to varying degrees. The habitats present at the proposed mine site are overwhelmingly indigenous and have a very high degree of intactness reflecting their lack of human disturbance. Previous surveys have shown that some trees in the area are more than 500 years old. Habitats affected by the proposed access road are less intact and include exotic pasture as well as regenerating shrubland and forest. Te Kuha is not part of the Department of Conservation’s Buller Coal Plateaux priority site and is unlikely to receive management for that reason. -

Phytochemistry and Biological Properties of Drimys Winteri JR Et G. Forster Var Chilensis (DC) A
BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión / Review Phytochemistry and biological properties of Drimys winteri JR et G. Forster var chilensis (DC) A. [Fitoquímica y propiedades biológicas de Drimys winteri JR et G. Forster var chilensis (DC) A.] Orlando Muñoz1, Jorge Tapia-Merino2, Wolf Nevermann, & Aurelio San-Martín3 1Departamento de Quimica, Facultad de Ciencias, Universidad de Chile, Ñuñoa, Santiago, Chile 2Departamento de Ciencias Químicas y Biológicas, Facultad de Salud, Universidad Bernardo OHiggins, Santiago, Chile 3Departamento de Ciencias y Recursos Naturales, Facultad de Ciencias, Universidad de Magallanes, Punta Arenas, Chile Abstract: Drimys winteri JR et G. Forster var chilensis (DC) A. is a tree native to central and southern Chile. Also it found in part of Argentina. It is abundant in wet swampy localities from sea level to an altitude of 1700 m. This tree is sacred for the Mapuche culture; it is used in folk medicine in such as Reviewed by: inflammatory and painful processes. Phytochemical studies have demonstrated that this plant contains Arnaldo Bandoni Universidad de Buenos Aires mainly sesquiterpenes of the drimane type, flavonoids, essential oils, phytosterols and some lignans. Argentina These drimanes have attracted interest because of their antifeedant, plant growth regulation, cytotoxic, antimicrobial and insecticidal properties. The objective of this review is to establish clearly the Ali Parlar phytochemistry and biological activity of Drimys winteri JR et G. Forster var chilensis (DC) A. Articles Adiyaman University based on other varieties are not considered. Turkey Keywords: Drimys winteri; Sesquiterpenes; Essential oils; Lignans; Flavonoids; Biological properties. -

9. a 10 Year Trial with South American Trees and Shrubs with Special
9. A 10 year trial with SouthAmerican trees and shrubswith specialregard to the Ir,lothofaglzsspp. I0 6ra royndir vid suduramerikonskumtroum og runnum vid serligumatliti at Nothofagw-slogum SarenOdum Abstract The potential of the ligneous flora of cool temperate South America in arboriculture in the Faroe Isles is elucidated through experimental planting of a broad variety of speciescollected on expeditions to Patagonia and Tierra del Fuego 1975 andl9T9.Particular good results have been obtained with the southernmost origins of Nothofagus antarctica, N. betuloides, and N. pumilio, of which a total of 6.500 plants were directly transplanted from Tierra del Fuego to the Faroe Isles in 1979. Soren Odum, Royal Vet.& Agric. IJniv., Arboretum, DK-2970 Horsholm, Denmark. Introduction As a student of botany at the University of CopenhagenI got the opportunity to get a job for the summer 1960as a member of the team mapping the flora of the Faroe Isles (Kjeld Hansen 1966). State geologist of the Faroe Isles and the Danish Geological Survey, J6annesRasmussen, provided working facilities for the team at the museum, and also my co-student,J6hannes J6hansen participated in the field. This stay and work founded my still growing interest in the Faroese nature and culture, and the initial connections between the Arboretum in Horsholm and Tbrshavn developed from this early contact with J6annesRasmussen and J6hannes J6hansen. On our way back to Copenhagen in 1960 onboard "Tjaldur", we called on Lerwick, Shetland, where I saw Hebe and Olearia in some gardens. This made it obvious to me, that if the Faroe Isles for historical reasonshad been more or less British rather than Nordic, the gardensof T6rshavn would, no doubt, have been speckledwith genera from the southern Hemisphere and with other speciesand cultivars nowadays common in Scottish nurseries and gardens. -

1992 New Zealand Botanical Society President: Dr Eric Godley Secretary/Treasurer: Anthony Wright
NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 30 DECEMBER 1992 New Zealand Botanical Society President: Dr Eric Godley Secretary/Treasurer: Anthony Wright Committee: Sarah Beadel, Ewen Cameron, Colin Webb, Carol West Address: New Zealand Botanical Society C/- Auckland Institute & Museum Private Bag 92018 AUCKLAND Subscriptions The 1993 ordinary and institutional subs are $14 (reduced to $10 if paid by the due date on the subscription invoice). The 1993 student sub, available to full-time students, is $7 (reduced to $5 if paid by the due date on the subscription invoice). Back issues of the Newsletter are available at $2.50 each - from Number 1 (August 1985) to Number 30 (December 1992). Since 1986 the Newsletter has appeared quarterly in March, June, September and December. New subscriptions are always welcome and these, together with back issue orders, should be sent to the Secretary/Treasurer (address above). Subscriptions are due by 28 February of each year for that calendar year. Existing subscribers are sent an invoice with the December Newsletter for the next year's subscription which offers a reduction if this is paid by the due date. If you are in arrears with your subscription a reminder notice comes attached to each issue of the Newsletter. Deadline for next issue The deadline for the March 1993 issue (Number 31) is 26 February 1993. Please forward contributions to: Bruce & Beverley Clarkson, Editors NZ Botanical Society Newsletter 7 Lynwood Place HAMILTON Cover illustration Asplenium pauperequitum (Aspleniaceae). Drawn by Lesley Alexander from specimens and photographs taken on the Poor Knights Islands. Lesley is completing a BA Hons Graphic Design at Middlesex Polytechnic, England, specialising in scientific illustration. -
M23 Tree History Workbook Pub 2018
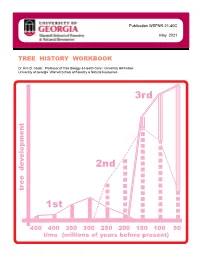
Publication WSFNR-21-40C May 2021 TREE HISTORY WORKBOOK Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources 3rd 2nd tree development 1st 450 400 350 300 250 200 150 100 50 time (millions of years before present) This workbook is an educational product designed for helping people interested in trees to appreciate and understand how modern trees developed across long time periods. This product is a synthesis and integration of research and educational concepts regarding tree evolution and species inception. This educational workbook was designed for awareness building and foundation training of professional tree health care providers. At the time it was finished, this workbook contained educational materials and models concerning tree development thought by the author to provide the best means for considering the most basic understandings of time impacts on tree species creation, extinction, and tree biological and mechanical fundamentals. The University of Georgia, the Warnell School of Forestry & Natural Resources, and the author are not responsible for any errors, omissions, misinterpretations, or misapplications stemming from this educational product. The author assumed all users would have some basic tree biological, structural, and ecological background. This product was not designed, nor is suited, as a literature or research review. Please use the selected literature section at the back of this workbook to find scientific sources. This publication is copyrighted by the author. This educational product is only for noncommercial, nonprofit use and may not be copied or reproduced in whole or in part, by any means, in any format, or in any media including electronic forms, without explicit written permission of the author.