12939.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tasmannia Lanceolata
ASPECTS OF LEAF AND EXTRACT PRODUCTION from Tasmannia lanceolata by Chris Read, B. Agr.Sc. Tas. Submitted in fulfillment of the requirements for the Degree of Doctor of Philosophy University of Tasmania, Hobart December 1995 ' s~, ... ~~ \ ·'(11 a_C\14 \t\J. \I ' This thesis contains no material which has been accepted for the award of any other degree or diploma in any University, and to the best of my knowledge, contains no copy or paraphrase of material previously written or published by any other person except where due reference is given in the text. University of Tasmania HOBART March 1996 This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968 University of Tasmania HOBART March 1996 Abstract This thesis examines several aspects of the preparation, extraction and analysis of solvent soluble compounds from leaf material of Tasmannia lanceolata and reports a preliminary survey of extracts of some members of the natural population of the species in Tasmania. A major constituent of these extracts, polygodial, was shown to be stored within specialised idioblastic structures scattered throughout the mesophyll, and characterised by distinctive size and shape, and a thickened wall. The contents of these cells were sampled directly, analysed and compared with the composition of extracts derived from ground, dry whole leaf. This result was supported by spectroscopic analysis of undisturbed oil cells in whole leaf tissue. In a two year field trial, the progressive accumulation of a number of leaf extract constituents (linalool, cubebene, caryophyllene, germacrene D, bicyclogermacrene, cadina-1,4 - diene, aristolone and polygodial) during the growth flush was followed by a slow decline during the subsequent dormant season. -

And According to Bailey & (1943)
BLUMEA 24 (1978) 521—525 The Winteraceae pf the Old World. III. Notes on the ovary of Takhtajania W. Vink Rijksherbarium, Leiden, Netherlands INTRODUCTION Very recently Baranova & Leroy (Leroy, 1978) published a new genus Takhta- jania to accomodate the aberrant Bubbia perrieri Capuron. The outstanding characters of this genus are the anomocytic stomatal apparatus (Baranova, 1972; Bongers, 1973) and the unilocular bicarpellate ovary (Leroy, 1977, 1978). work the Winteraceae, also studied the of Bub- During my on I single specimen bia perrieri in existence. The late Capuron told me that he had tried to collect additional specimens but that he had not succeeded in doing so; according to him the type locality was completely deforested. In view of the scarcity of the material it was considered relevant to publish some additional notes without delay. MATERIAL AND METHODS I studied three ovaries of the type specimen (Perrier de la Bdthie 10158). One was cut longitudinally, slightly outside the plane of symmetry; another was cut and its half These drawn transversally upper again longitudinally. parts were (figs. 2 and 3) and then cleared according to Bailey & Nast (1943) (figs 5 and third boiled and then sectioned 6). The ovary was serially (fig. 4). OBSERVATIONS One of the ovaries was taken from a flowerbud of which the closed petals were 57 mm high. The diagram of this flowerbud is drawn in fig. 1. The 2 slightly ruptured calyx showed three putative original apices ± alternating with the three bracteoles at the base of the pedicel. The petals are all free. The ovary is its and the flattened, has a longitudinal groove on narrow sides, highest sta- mens are inserted adjoining its broadest sides. -

<I>Winteraceae</I>
Blumea 59, 2014: 155–162 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE http://dx.doi.org/10.3767/000651914X686022 The Winteraceae of the Old World. VII. Zygogynum in the Solomon Islands (incl. Bougainville) W. Vink1 Key words Abstract A review of the character distribution in the Zygogynum representatives from the Solomon Islands (and Bougainville) shows a rather wide variability in ranges of numbers and sizes, often with distinct differences between Solomon Islands islands or altitudinal ranges. However, the morphology is rather uniform. The four species recognised by A.C. Smith Winteraceae had to be synonymised, but a new taxon deserved recognition. The name Bubbia is explained. Zygogynum Published on 19 December 2014 INTRODUCTION CHARACTERS AND THEIR VARIABILITY The first Zygogynum species described from the Solomon Is- Leaves lands is Bubbia haplopus B.L.Burtt (1936). In his monumental Except for size, the leaves are very uniform with the blade works on the Papuasian plants, Smith (1942) transferred this chartaceous, the midrib impressed above and prominent below. species from Bubbia to the other Van Tieghem genus, Belliolum, On the lower side of the blade the grey to white alveolar mate- and described three more species from this area. The materials rial covers only the stomata or unites these into small strings available to Smith were, however, very incomplete, and several or clusters; the stomata are also present over the secondary characters could not be established; next to leaf size, minor nerves, or less dense to lacking over the thickest parts of the differences had to be used to define these taxa. -

Phytochemistry and Biological Properties of Drimys Winteri JR Et G. Forster Var Chilensis (DC) A
BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión / Review Phytochemistry and biological properties of Drimys winteri JR et G. Forster var chilensis (DC) A. [Fitoquímica y propiedades biológicas de Drimys winteri JR et G. Forster var chilensis (DC) A.] Orlando Muñoz1, Jorge Tapia-Merino2, Wolf Nevermann, & Aurelio San-Martín3 1Departamento de Quimica, Facultad de Ciencias, Universidad de Chile, Ñuñoa, Santiago, Chile 2Departamento de Ciencias Químicas y Biológicas, Facultad de Salud, Universidad Bernardo OHiggins, Santiago, Chile 3Departamento de Ciencias y Recursos Naturales, Facultad de Ciencias, Universidad de Magallanes, Punta Arenas, Chile Abstract: Drimys winteri JR et G. Forster var chilensis (DC) A. is a tree native to central and southern Chile. Also it found in part of Argentina. It is abundant in wet swampy localities from sea level to an altitude of 1700 m. This tree is sacred for the Mapuche culture; it is used in folk medicine in such as Reviewed by: inflammatory and painful processes. Phytochemical studies have demonstrated that this plant contains Arnaldo Bandoni Universidad de Buenos Aires mainly sesquiterpenes of the drimane type, flavonoids, essential oils, phytosterols and some lignans. Argentina These drimanes have attracted interest because of their antifeedant, plant growth regulation, cytotoxic, antimicrobial and insecticidal properties. The objective of this review is to establish clearly the Ali Parlar phytochemistry and biological activity of Drimys winteri JR et G. Forster var chilensis (DC) A. Articles Adiyaman University based on other varieties are not considered. Turkey Keywords: Drimys winteri; Sesquiterpenes; Essential oils; Lignans; Flavonoids; Biological properties. -
M23 Tree History Workbook Pub 2018
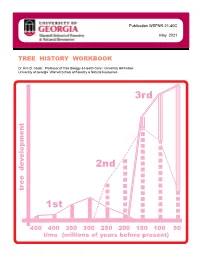
Publication WSFNR-21-40C May 2021 TREE HISTORY WORKBOOK Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources 3rd 2nd tree development 1st 450 400 350 300 250 200 150 100 50 time (millions of years before present) This workbook is an educational product designed for helping people interested in trees to appreciate and understand how modern trees developed across long time periods. This product is a synthesis and integration of research and educational concepts regarding tree evolution and species inception. This educational workbook was designed for awareness building and foundation training of professional tree health care providers. At the time it was finished, this workbook contained educational materials and models concerning tree development thought by the author to provide the best means for considering the most basic understandings of time impacts on tree species creation, extinction, and tree biological and mechanical fundamentals. The University of Georgia, the Warnell School of Forestry & Natural Resources, and the author are not responsible for any errors, omissions, misinterpretations, or misapplications stemming from this educational product. The author assumed all users would have some basic tree biological, structural, and ecological background. This product was not designed, nor is suited, as a literature or research review. Please use the selected literature section at the back of this workbook to find scientific sources. This publication is copyrighted by the author. This educational product is only for noncommercial, nonprofit use and may not be copied or reproduced in whole or in part, by any means, in any format, or in any media including electronic forms, without explicit written permission of the author. -

Djvu Document
Cenomanian Angiosperm Leaf Megafossils, Dakota Formation, Rose Creek Locality, Jefferson County, Southeastern Nebraska By GARLAND R. UPCHURCH, JR. and DAVID L. DILCHER U.S. GEOLOGICAL SURVEY BULLETIN 1915 DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. UNITED STATES GOVERNMENT PRINTING OFFICE: 1990 For sale by the Books and Open-File Reports Section U.S. Geological Survey Federal Center Box 25425 Denver. CO 80225 Library of Congress Cataloging-in-Publication Data Upchurch, Garland R. Cenomanian angiosperm leaf megafossils, Dakota Formation, Rose Creek locality, Jefferson County, southeastern Nebraska / by Garland R. Upchurch, Jr., and David L. Dilcher. p. cm.-(U.S. Geological Survey bulletin; 1915) Includes bibliographical references. Supt. of Docs. no.: 1 19.3:1915. 1. Leaves, Fossil-Nebraska-Jefferson County. 2. Paleobotany-Cretaceous. 3. Paleobotany-Nebraska-Jefferson County. I. Dilcher, David L. II. Title. III. Series. QE75.B9 no. 1915 [QE983] 557.3 s-dc20 90-2855 [561'.2]CIP CONTENTS Abstract 1 Introduction 1 Acknowledgments 2 Materials and methods 2 Criteria for classification 3 Geological setting and description of fossil plant locality 4 Floristic composition 7 Evolutionary considerations 8 Ecological considerations 9 Key to leaf types at Rose Creek 10 Systematics 12 Magnoliales 12 Laurales 13 cf. Illiciales 30 Magnoliidae order -

Intercontinental Long-Distance Dispersal of Canellaceae from the New to the Old World Revealed by a Nuclear Single Copy Gene and Chloroplast Loci
Molecular Phylogenetics and Evolution 84 (2015) 205–219 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Intercontinental long-distance dispersal of Canellaceae from the New to the Old World revealed by a nuclear single copy gene and chloroplast loci Sebastian Müller a,1, Karsten Salomo a,1, Jackeline Salazar b, Julia Naumann a, M. Alejandra Jaramillo c, ⇑ Christoph Neinhuis a, Taylor S. Feild d,2, Stefan Wanke a, ,2 a Technische Universität Dresden, Institut für Botanik, Zellescher Weg 20b, 01062 Dresden, Germany b Escuela de Biología, Universidad Autónoma de Santo Domingo (UASD), C/Bartolomé Mitre, Santo Domingo, Dominican Republic c Centro de Investigación para el Manejo Ambiental y el Desarrollo, Cali, Colombia d Centre for Tropical Biodiversity and Climate Change, College of Marine and Environmental Science, Townsville 4810, Campus Townsville, Australia article info abstract Article history: Canellales, a clade consisting of Winteraceae and Canellaceae, represent the smallest order of magnoliid Received 10 July 2014 angiosperms. The clade shows a broad distribution throughout the Southern Hemisphere, across a diverse Revised 16 December 2014 range of dry to wet tropical forests. In contrast to their sister-group, Winteraceae, the phylogenetic rela- Accepted 17 December 2014 tions and biogeography within Canellaceae remain poorly studied. Here we present the phylogenetic Available online 9 January 2015 relationships of all currently recognized genera of Canellales with a special focus on the Old World Canellaceae using a combined dataset consisting of the chloroplast trnK-matK-trnK-psbA and the nuclear Keywords: single copy gene mag1 (Maigo 1). Within Canellaceae we found high statistical support for the mono- Canellales phyly of Warburgia and Cinnamosma. -

Exospermum Stipitatum (Winteraceae): Observations on Wood, Leaves, Flowers, Pollen, and Fruit Sherwin Carlquist
Aliso: A Journal of Systematic and Evolutionary Botany Volume 10 | Issue 2 Article 7 1982 Exospermum stipitatum (Winteraceae): Observations on Wood, Leaves, Flowers, Pollen, and Fruit Sherwin Carlquist Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Carlquist, Sherwin (1982) "Exospermum stipitatum (Winteraceae): Observations on Wood, Leaves, Flowers, Pollen, and Fruit," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 10: Iss. 2, Article 7. Available at: http://scholarship.claremont.edu/aliso/vol10/iss2/7 ALISO 10(2) , 1982 , pp. 277-289 EXOSPERMUM STIPITATUM (WINTERACEAE): OBSERVATIONS ON WOOD, LEAYES, FLOWERS, POLLEN, AND FRUIT Sherwin Carlquist Introduction The studies of Bailey and Nast (1943a, 1943b, 1944a, 1944b) reported on Exospermum van Tiegh. as part of a series of studies on anatomy of Win teraceae. Since that time, a study on flowers of the genus by Sampson and Tucker (1978) has appeared. That study was based upon liquid-preserved material and utilized scanning electron microscopy. Sampson and Tucker demonstrated that ovule placement in Exospermum is not laminar, as had been alleged. Bongers (1973) included Exospermum in a survey of foliar epidermis in Winteraceae. In 1978, I was able to collect excellent material of Exospermum stipitatum (Baill.) van Tiegh. ex Pilg. from middle elevations of Mt. Panie, New Cal edonia. Trees were in flower and permitted observations on details of flower opening and pollen presentation. From this material, liquid-preserved spec imens upon which anatomical observations could be made were prepared. Wood of these trees was also obtained at that time. Wood anatomy of Exospermum has not been described hitherto. -

The Family Winteraceae Author(S): J
The Family Winteraceae Author(s): J. Hutchinson Source: Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew), Vol. 1921, No. 5 (1921), pp. 185-191 Published by: Springer on behalf of Royal Botanic Gardens, Kew Stable URL: http://www.jstor.org/stable/4118626 Accessed: 26-06-2016 14:32 UTC Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Royal Botanic Gardens, Kew, Springer are collaborating with JSTOR to digitize, preserve and extend access to Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew) This content downloaded from 198.91.37.2 on Sun, 26 Jun 2016 14:32:19 UTC All use subject to http://about.jstor.org/terms 185 by felling it and stripping it of its bark, (a) unless the substance could be extracted economically from its leaves or annual prunings, (b) or unless some means of paring off the bark without destroying the cambium could be devised, (c) or unless the tree were amenable to the coppice system. The last possibility is perhaps the most likely. In this case the peeled poles might form a subsidiary source of revenue. 4. High cost of land and wages. In conclusion the writer would like to take this opportunity of expressing his thanks to Prof. -

An Early Record of a Vesselless Angiosperm from the Middle Cenomanian of the Envigne Valley (Vienne, Western France) A
An Early Record of a Vesselless Angiosperm from the Middle Cenomanian of the Envigne Valley (Vienne, Western France) A. Boura, G. Saulnier, D. de Franceschi, B. Gomez, V. Daviero-Gomez, D. Pons, Géraldine Garcia, N. Robin, J-M. Boiteau, Xavier Valentin To cite this version: A. Boura, G. Saulnier, D. de Franceschi, B. Gomez, V. Daviero-Gomez, et al.. An Early Record of a Vesselless Angiosperm from the Middle Cenomanian of the Envigne Valley (Vienne, Western France). IAWA Journal, Brill publishers, 2019, 40 (3), pp.2–21. 10.1163/22941932-40190238. hal-02871851v1 HAL Id: hal-02871851 https://hal.archives-ouvertes.fr/hal-02871851v1 Submitted on 5 Mar 2021 (v1), last revised 12 May 2021 (v2) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Running title: Cenomenian Vesselless Angiosperm 2 Accepted for publication on … January 2019 3 An early record of a vesselless angiosperm from the middle Cenomanian of the Envigne valley (Vienne, 4 Western France) 5 6 A. Boura1*, G. Saulnier1, D. De Franceschi1, B. Gomez2, V. Daviero-Gomez2, D. Pons1, 7 G. Garcia3, N. Robin1, J-M. Boiteau4 and X. Valentin3,5 8 9 1 CR2P, UMR7207, MNHN, Sorbonne Université, CNRS, 57 rue Cuvier, CP 48, F-75005, Paris, France 10 2 LGL, UMR 5276, Université Lyon 1, Villeurbanne 69622, France 11 3 PALEVOPRIM, UMR7262 CNRS INEE, Université de Poitiers, 6, rue Michel-Brunet, 86073 Poitiers cedex, 12 France. -

Tasmannia Lanceolata.14-06-17
Tasmannia lanceolata Tasmannia lanceolata (syn. Drimys lanceolata). Family: Winteraceae Common names: Mountain pepper, Tasmanian pepperberry, Cornish pepper leaf (UK). Tasmannia lanceolata Mt Buffalo, Vic (E.Bennetto) Tasmannia lanceolata is a shrub native to woodlands and cool temperate rainforest of south-eastern Australia. The shrub varies from 2 to 10 m high. The aromatic leaves are lanceolate to narrow-elliptic, 4–12 cm long, and 0.7– 2.0 cm wide, with a distinctly pale undersurface. Stems are quite red in colour. The small cream or white flowers appear in summer and are followed by black, globose, two-lobed berries 5–8 mm wide, which appear in autumn. There are separate male and female plants. Originally described by French botanist Jean Louis Marie Poiret, it gained its current name in 1969 by A.C. Smith. It had been known for many years as Drimys lanceolata. Uses The leaf and berry are used as a spice, typically dried. Mountain pepper was used as a colonial pepper substitute. More recently, it has become popularised as bushfood condiment. It can be added to curries, cheeses, and alcoholic beverages. It is exported to Japan to flavour wasabi. Dried T. lanceolata berries and leaves have strong antimicrobial activity against food spoilage organisms. It also has high antioxidant activity. Used in colonial medicine as a substitute for Winter's bark (stomachic) , it was also used for treating scurvy. It can be grown as a garden plant, and its berries attract birds. Currawongs are among those which feed on them. It can be propagated from cuttings or seed, and can grow in a well-drained acidic soil with some shade, but is sensitive to Phytophthora cinnamomi. -

The Woody Planet: from Past Triumph to Manmade Decline
plants Review The Woody Planet: From Past Triumph to Manmade Decline Laurence Fazan 1, Yi-Gang Song 2,3 and Gregor Kozlowski 1,3,4,* 1 Department of Biology and Botanical Garden, University of Fribourg, Chemin du Musée 10, 1700 Fribourg, Switzerland; [email protected] 2 Eastern China Conservation Center for Wild Endangered Plant Resources, Shanghai Chenshan Botanical Garden, Chenhua Road No.3888, Songjiang, Shanghai 201602, China; [email protected] 3 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences, Chenhua Road No.3888, Songjiang, Shanghai 201602, China 4 Natural History Museum Fribourg, Chemin du Musée 6, 1700 Fribourg, Switzerland * Correspondence: [email protected]; Tel.: +41-26-300-88-42 Received: 6 November 2020; Accepted: 16 November 2020; Published: 17 November 2020 Abstract: Woodiness evolved in land plants approximately 400 Mya, and very soon after this evolutionary invention, enormous terrestrial surfaces on Earth were covered by dense and luxurious forests. Forests store close to 80% of the biosphere’s biomass, and more than 60% of the global biomass is made of wood (trunks, branches and roots). Among the total number of ca. 374,000 plant species worldwide, approximately 45% (138,500) are woody species—e.g., trees, shrubs or lianas. Furthermore, among all 453 described vascular plant families, 191 are entirely woody (42%). However, recent estimations demonstrate that the woody domination of our planet was even greater before the development of human civilization: 1.4 trillion trees, comprising more than 45% of forest biomass, and 35% of forest cover disappeared during the last few thousands of years of human dominance on our planet.