Responses of North American Papilio Troilus and P. Glaucus to Potential Hosts from Australia J
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

TML Propagation Protocols
PROPAGATION PROTOCOLS This document is intended as a guide for Tamborine Mountain Landcare members who wish to assist our regeneration projects by growing some of the plants needed. It is a work in progress so if you have anything to add to the protocols – for example a different but successful way of propagating and growing a particular plant – then please give it to Julie Lake so she can add it to the document. The idea is that our shared knowledge and experience can become a valuable part of TML's intellectual property as well as a useful source of knowledge for members. As there are many hundreds of plants native to Tamborine Mountain, the protocols list will take a long time to complete, with growing information for each plant added alphabetically as time permits. While the list is being compiled by those members with competence in this field, any TML member with a query about propagating a particular plant can post it on the website for other me mb e r s to answer. To date, only protocols for trees and shrubs have been compiled. Vines and ferns will be added later. Fruiting times given are usual for the species but many rainforest plants flower and fruit opportunistically, according to weather and other conditions unknown to us, thus fruit can be produced at any time of year. Finally, if anyone would like a copy of the protocols, contact Julie on [email protected] and she’ll send you one. ………………….. Growing from seed This is the best method for most plants destined for regeneration projects for it is usually fast, easy and ensures genetic diversity in the regenerated landscape. -

Diet of the White-Headed Pigeon Columba Leucomela Near Lismore, Northern New South Wales: Fruit, Seeds, Flower Buds, Bark and Grit
Corella, 2011, 35(3): 107-111 Diet of the White-headed Pigeon Columba leucomela near Lismore, northern New South Wales: fruit, seeds, flower buds, bark and grit D. G. Gosper 39 Azure Avenue, Balnarring, Victoria 3926, Australia Received: 27 May 2010 Gut contents of 18 White-headed Pigeons Columba leucomela, found dead over a four-year period near Lismore in northern New South Wales, comprised fruits and seeds of the invasive plant Camphor Laurel Cinnamomum camphora almost exclusively. Birds frequently ingested Melaleuca quinquenervia bark, which, as far as I am aware, constitutes the fi rst record of consumption of bark in the Columbidae, prompting some interesting hypotheses. It is suggested that bark ingestion may counter potential adverse effects from a diet dominated by Camphor Laurel fruits and seeds, which are reputed to contain toxins. Incidental records of consumption of fl ower buds of indigenous plants and insects (the fi rst such records for this species), and regular drinking from man-made structures such as roof guttering on buildings are detailed. INTRODUCTION surrounding area is a mixture of pasture, remnant riparian rainforest and regrowth forest with extensive areas dominated The White-headed Pigeon Columba leucomela is known to by woody weed species, notably Camphor Laurel and Broad- feed on the fruits and seeds of a number of fl eshy-fruited invasive leaved Privet Ligustrum lucidum, and several house gardens. plants, notably Camphor Laurel Cinnamomum camphora, which has become an important seasonal food source for this species Dead pigeons were collected and crop and gizzard contents in northern New South Wales (NSW) (Frith 1982; Recher and removed during subsequent dissection. -

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

Cassytha) and Insights Into the Plastid Phylogenomics of Lauraceae
GBE Plastome Evolution in the Sole Hemiparasitic Genus Laurel Dodder (Cassytha) and Insights into the Plastid Phylogenomics of Lauraceae Chung-Shien Wu1,†, Ting-Jen Wang1,†,Chia-WenWu1, Ya-Nan Wang2, and Shu-Miaw Chaw1,* 1Biodiversity Research Center, Academia Sinica, Taipei 11529, Taiwan 2School of Forestry and Resource Conservation, Nation Taiwan University, Taipei 10617, Taiwan *Corresponding author: E-mail: [email protected]. †These authors contributed equally to this work. Accepted: September 6, 2017 Data deposition: This project has been deposited at DDBJ under the accession numbers LC210517, LC212965, LC213014, and LC228240. Abstract To date, little is known about the evolution of plastid genomes (plastomes) in Lauraceae. As one of the top five largest families in tropical forests, the Lauraceae contain many species that are important ecologically and economically. Lauraceous species also provide wonderful materials to study the evolutionary trajectory in response to parasitism because they contain both nonparasitic and parasitic species. This study compared the plastomes of nine Lauraceous species, including the sole hemiparasitic and herbaceous genus Cassytha (laurel dodder; here represented by Cassytha filiformis). We found differential contractions of the canonical inverted repeat (IR), resulting in two IR types present in Lauraceae. These two IR types reinforce Cryptocaryeae and Neocinnamomum— Perseeae–Laureae as two separate clades. Our data reveal several traits unique to Cas. filiformis, including loss of IRs, loss or pseudogenization of 11 ndh and rpl23 genes, richness of repeats, and accelerated rates of nucleotide substitutions in protein- coding genes. Although Cas. filiformis is low in chlorophyll content, our analysis based on dN/dS ratios suggests that both its plastid house-keeping and photosynthetic genes are under strong selective constraints. -
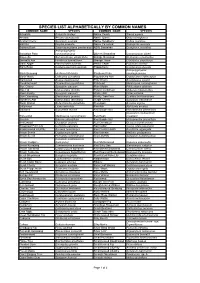
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

EPBC Protected Matters Database Search Results
FLORA AND FAUNA TECHNICAL REPORT Gold Coast Quarry EIS ATTACHMENT A – EPBC Protected Matters Database Search Results April 2013 Cardno Chenoweth 71 EPBC Act Protected Matters Report This report provides general guidance on matters of national environmental significance and other matters protected by the EPBC Act in the area you have selected. Information on the coverage of this report and qualifications on data supporting this report are contained in the caveat at the end of the report. Information about the EPBC Act including significance guidelines, forms and application process details can be found at http://www.environment.gov.au/epbc/assessmentsapprovals/index.html Report created: 01/06/12 14:33:07 Summary Details Matters of NES Other Matters Protected by the EPBC Act Extra Information Caveat Acknowledgements This map may contain data which are ©Commonwealth of Australia (Geoscience Australia), ©PSMA 2010 Coordinates Buffer: 6.0Km Summary Matters of National Environment Significance This part of the report summarises the matters of national environmental significance that may occur in, or may relate to, the area you nominated. Further information is available in the detail part of the report, which can be accessed by scrolling or following the links below. If you are proposing to undertake an activity that may have a significant impact on one or more matters of national environmental significance then you should consider the Administrative Guidelines on Significance - see http://www.environment.gov.au/epbc/assessmentsapprovals/guidelines/index.html World Heritage Properties: None National Heritage Places: None Wetlands of International 1 Great Barrier Reef Marine Park: None Commonwealth Marine Areas: None Threatened Ecological Communities: 1 Threatened Species: 57 Migratory Species: 27 Other Matters Protected by the EPBC Act This part of the report summarises other matters protected under the Act that may relate to the area you nominated. -

Attachment E - Desktop Searches
Attachment E - Desktop searches EPBC Act Protected Matters Report This report provides general guidance on matters of national environmental significance and other matters protected by the EPBC Act in the area you have selected. Information on the coverage of this report and qualifications on data supporting this report are contained in the caveat at the end of the report. Information is available about Environment Assessments and the EPBC Act including significance guidelines, forms and application process details. Report created: 11/06/20 13:02:49 Summary Details Matters of NES Other Matters Protected by the EPBC Act Extra Information Caveat Acknowledgements This map may contain data which are ©Commonwealth of Australia (Geoscience Australia), ©PSMA 2010 Coordinates Buffer: 20.0Km Summary Matters of National Environmental Significance This part of the report summarises the matters of national environmental significance that may occur in, or may relate to, the area you nominated. Further information is available in the detail part of the report, which can be accessed by scrolling or following the links below. If you are proposing to undertake an activity that may have a significant impact on one or more matters of national environmental significance then you should consider the Administrative Guidelines on Significance. World Heritage Properties: None National Heritage Places: None Wetlands of International Importance: None Great Barrier Reef Marine Park: None Commonwealth Marine Area: None Listed Threatened Ecological Communities: 4 Listed Threatened Species: 26 Listed Migratory Species: 16 Other Matters Protected by the EPBC Act This part of the report summarises other matters protected under the Act that may relate to the area you nominated. -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

(Lepidoptera: Papilionidae) of Kerala Part of Western Ghats Usin
Journal of Entomology and Zoology Studies 2014; 2 (4): 72-77 ISSN 2320-7078 Taxonomic segregation of the Swallowtails of the JEZS 2014; 2 (4): 72-77 © 2014 JEZS genus Graphium (Lepidoptera: Papilionidae) of Received: 23-06-2014 Accepted: 17-07-2014 Kerala part of Western Ghats using morphological V.S. Revathy characters of external genitalia Entomology Department, Forest Health Division, Kerala Forest V.S. Revathy and George Mathew Research Institute, Peechi, Kerala- 680635 Abstract George Mathew Studies on the genitalia of four species of Papilionids belonging to the tribe Leptocercini were made. The Entomology Department, Forest structure of vinculum, uncus, valvae and phallus of the male genitalia and the bursa, ductus and ovipositor Health Division, Kerala Forest of the female were found to be useful in taxonomic segregation of these butterflies. This highlights the Research Institute, Peechi, Kerala- extreme practical importance of external genitalic structures in the identification of these butterflies and 680635 improves upon earlier characters for generic and specific determinations based mainly on the wing venation, size and shape of palpi, and frons. Keywords: Taxonomy, Papilionidae, Lepidoptera, Graphium, Western Ghats 1. Introduction The Western Ghats constitute a mountain range along the western side of India. It is acclaimed as World Heritage Site by UNESCO and is one of the world’s eight “hottest hotspots" of biological diversity. Southern Western Ghats extending from the Agasthamalai to Palghat Gap has highest butterfly diversity with maximum Endemics. Thirty six species of butterflies are reported to be endemic to the Ghat and among the butterfly genera, the genus Parantirrhoea is exclusively [11] endemic to this region . -

Invasion and Management of a Woody Plant, Lantana Camara L., Alters Vegetation Diversity Within Wet Sclerophyll Forest in Southeastern Australia
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Ben Gooden University of Wollongong, [email protected] Kris French University of Wollongong, [email protected] Peter J. Turner Department of Environment and Climate Change, NSW Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Gooden, Ben; French, Kris; and Turner, Peter J.: Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia 2009. https://ro.uow.edu.au/scipapers/4953 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Abstract Plant invasions of natural communities are commonly associated with reduced species diversity and altered ecosystem structure and function. This study investigated the effects of invasion and management of the woody shrub Lantana camara (lantana) in wet sclerophyll forest on the south-east coast of Australia. The effects of L. camara invasion and management on resident vegetation diversity and recruitment were determined as well as if invader management initiated community recovery. Vascular plant species richness, abundance and composition were surveyed and compared across L. -

Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp.