Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
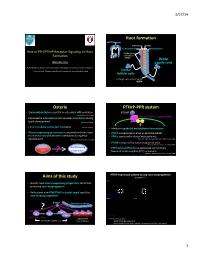
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Benign Cementoblastoma Associated with an Unerupted Third Molar - a Case Report
CORE Metadata, citation and similar papers at core.ac.uk Provided by Directory of Open Access Journals BENIGN CEMENTOBLASTOMA ASSOCIATED WITH AN UNERUPTED THIRD MOLAR - A CASE REPORT J.Dinakar* M.S.Senthil Kumar** Shiju Mathew Jacob*** *Professor & HOD, Department of Oral Pathology, ** Reader, *** Lecturer, Department of Oral Surgery, Sri Ramakrishna Dental College and Hospital, Coimbatore, Tamilnadu, India. ABSTRACT: Cementoblastoma is a rare odontogenic tumor derived from odontogenic ectomesenchyme of cementoblast origin that forms cementum layer on the roots of a tooth. A case report is presented of a patient treated with surgical excision of Cementoblastoma associated with an unerupted infected right lower third molar tooth. Key words: Cementoblastoma, Odontogenic tumour, unerupted third molar. The cell of origin is cementoblast. INTRODUCTION: Clinically it causes bony expansion. The commonest site is the posterior region of Cementoblastoma is an odontogenic the mandible. In the radiograph it is seen tumor of ectomesenchymal origin. It is as large radiopaque mass associated with also called cementoma. They are large the root of the tooth. We report a case of bulbous mass of cementum or cementum- Benign Cementoblastoma from Sri like tissue on roots of teeth. Ramakrishna Dental College & Hospital, Coimbatore. restriction in opening the mouth and intra oral examination reveals a partially CASE REPORT: erupted third molar tooth with pus discharge. A panoramic radiograph A 41 year old man presented to our showed a radio-opaque, dense, department with a complaint of pain and amorphous, irregularly shaped mass swelling in the right lower half of the face. measuring 2.2 x 1.5cm attached with the Patient gave history of intermittent pain third molar (Fig 1,1a). -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Original Article BMP-2 Downregulation Is Involved in the Inhibition of Cementoblast Differentiation by Lithium
Int J Clin Exp Med 2016;9(2):823-830 www.ijcem.com /ISSN:1940-5901/IJCEM0017314 Original Article BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium Shang Gao1*, Xing-Fu Bao1*, Yu-Zhuo Wang1, Jing-Zheng Yi2, Min Hu1 1Department of Orthodontics, Stomatological Hospital of Jilin University, China; 2University of California in San Diego, USA. *Equal contributors. Received October 5, 2015; Accepted December 8, 2015; Epub February 15, 2016; Published February 29, 2016 Abstract: Cementum, which is formed by cementoblast, plays an important role in periodontal regeneration. Wnt signalling and BMP-2 are involved in the regulation of cementoblast differentiation, but little is known about the effect of lithium, an agonist of Wnt signalling, on cementoblast behaviour. In this research, OCCM-30 cementoblast was employed to investigate the influence of various concentrations of lithium on proliferation and differentiation. Cell metabolic assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, cell staining, alizarin red staining and alkaline phosphatase assay were performed. BMP-2 expression was also compared among different groups. Results showed no significant difference in proliferation, whereas differentiation was inhibited in the pres- ence of lithium. Given these results, along with previous reports, BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium. Keywords: Lithium, cementoblast, BMP-2 Introduction Bone morphogenetic protein 2 (BMP-2) is an autocrine and paracrine growth factor that is Cementum is a type of special mineralized tis- essential for the growth of mineralized tissue. sue covering tooth root that protects dental In osteoblasts, BMP-2 promotes the differentia- pulp from external stimulation. -

Dental Manifestations in Bariatric Patients – Review of Literature
www.scielo.br/jaos Dental manifestations in bariatric patients – review of literature Carolina Silveira BARBOSA1, Gabriel Salles BARBÉRIO1, Vinicius Rizzo MARQUES1, Vitor de Oliveira BALDO1, Marília Afonso Rabelo BUZALAF2, Ana Carolina MAGALHÃES3 1- Undergraduate student, Bauru School of Dentistry, University of São Paulo. 2- DDS, MSc, PhD, Full Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo. 3- DDS, MSc, PhD, Assistant Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo. Corresponding address: Ana Carolina Magalhães - Faculdade de Odontologia de Bauru - Universidade de São Paulo - Departamento de Ciências Biológicas - Al. Octávio Pinheiro Brisolla, 9-75 Bauru-SP 17012-901 (Brazil) - Phone: + 55 14 32358246 Fax: + 55 14 32343164 - e-mail: [email protected] Received: August 19, 2009 - Accepted: February 19, 2010 ABSTRACT he rate of bariatric surgery has significantly risen in the past decade as an increasing Tprevalence of extreme obesity can be observed. Although bariatric surgery is an effective therapeutic modality for extreme obesity, it is associated with risk factors affecting also oral health. Based on an overview of the current literature, this paper presents a summary of dental manifestations in bariatric patients. Bariatric surgeries are associated with an increased risk for gastro-esophageal reflux which in turn might account for the higher amount of carious and erosive lesions observed in bariatric patients. As a result, also dentin hypersensitivity might be observed more frequently. The current data indicate that recommended postsurgical meal patterns and gastric reflux might increase the risk for dental lesions, particularly in the presence of other risk factors, such as consumption of sweet-tasting foods and acidic beverages. -

Title Erupted Complex Odontoma Delayed Eruption of Permanent
Erupted complex odontoma delayed eruption of Title permanent molar Author(s) Ohtawa, Y; Ichinohe, S; Kimura, E; Hashimoto, S Journal Bulletin of Tokyo Dental College, 54(4): 251-257 URL http://hdl.handle.net/10130/5321 Right Description Posted at the Institutional Resources for Unique Collection and Academic Archives at Tokyo Dental College, Available from http://ir.tdc.ac.jp/ Bull Tokyo Dent Coll (2013) 54(4): 251–257 Case Report Erupted Complex Odontoma Delayed Eruption of Permanent Molar Yumi Ohtawa, Saori Ichinohe*, Eri Kimura* and Sadamitsu Hashimoto** Division of Special Needs Dentistry, Department of Oral Health Science, Tokyo Dental College, 2-9-18 Misaki-cho, Chiyoda-ku, Tokyo 101-0061, Japan * Division of Pediatric Dentistry, Department of Oral Health Science, Tokyo Dental College, 2-9-18 Misaki-cho, Chiyoda-ku, Tokyo 101-0061, Japan ** Department of Biology, Tokyo Dental College, 2-9-7 Kanda-surugadai, Chiyoda-ku, Tokyo 101-0062, Japan Received 26 April, 2013/Accepted for publication 10 June, 2013 Abstract Odontomas, benign tumors that develop in the jaw, rarely erupt into the oral cavity. We report an erupted odontoma which delayed eruption of the first molar. The patient was a 10-year-old Japanese girl who came to our hospital due to delayed eruption of the right maxillary first molar. All the deciduous teeth had been shed. The second premolar on the right side had erupted, but not the first molar. Slight inflammation of the alveolar mucosa around the first molar had exposed a tooth-like, hard tissue. Panoramic radiog- raphy revealed a radiopaque mass indicating a lesion approximately 1 cm in diameter. -

Developmental Biology of Cementum
Int. J. Dev. Biol. 45: 695-706 (2001) Review Developmental Biology of Cementum THOMAS G.H. DIEKWISCH* Allan G. Brodie Laboratory for Craniofacial Genetics, University of Illinois at Chicago, USA CONTENTS Origins of cementum - a scientific "whodunit" ........................................................................695 Loss of ameloblast continuity and insertion of mesenchymal cells from the dental follicle proper ................................................................................................697 Initial cementum matrix deposition by mesenchymal cells in proximity to non-secretory epithelial cells ...................................................................................699 Cementogenesis at the tooth cervix and at the cemento-enamel junction .............................700 Early removal of HERS from the root surface in humans as seen in the Gottlieb collection ..............................................................................................701 Role of amelogenins in cementogenesis ................................................................................702 Possible mechanism of cementoblast induction .....................................................................704 Summary ................................................................................................................................704 KEY WORDS: Cementum, Hertwig’s epithelial root sheath, Gottlieb, amelogenin, periodontium Tooth cementum is a bone-like mineralized tissue secreted by Origins of cementum - a scientific -

Mesenchymal Stem Cells and Tooth Engineering Peng Et Al
Mesenchymal Stem Cells and Tooth Engineering Peng et al. REVIEW Mesenchymal Stem Cells and Tooth Engineering Li Peng, Ling Ye, Xue-dong Zhou* State Key Laboratory of Oral Disease, Sichuan University, Chengdu, China Abstract multipotent stem cells which can differentiate into a variety Li Peng, Ling Ye, Xue-dong Zhou. Mesenchymal Stem Cells of cell types. The potential MSCs for tooth regeneration and Tooth Engineering. International Journal of Oral mainly include stem cells from human exfoliated Science, 1(1): 6–12, 2009 deciduous teeth (SHEDs), adult dental pulp stem cells (DPSCs), stem cells from the apical part of the papilla Tooth loss compromises human oral health. Although (SCAPs), stem cells from the dental follicle (DFSCs), several prosthetic methods, such as artificial denture and periodontal ligament stem cells (PDLSCs) and bone dental implants, are clinical therapies to tooth loss marrow derived mesenchymal stem cells (BMSCs). This problems, they are thought to have safety and usage time review outlines the recent progress in the mesenchymal issues. Recently, tooth tissue engineering has attracted stem cells used in tooth regeneration. more and more attention. Stem cell based tissue engineering is thought to be a promising way to replace the Keywords mesenchymal stem cell, tooth engineering, missing tooth. Mesenchymal stem cells (MSCs) are dental pulp stem cell Document code: A CLC number: Q813.1 Received Dec.18,2008; Revision accepted Jan.21,2009 Introduction can be divided into embryonic stem cells and adult stem cells. Differentiation and proliferation of A commonly applied definition of tissue engi- embryonic stem cells constitute the basis of animal neering, as stated by Langer and Vacanti, is “an development. -

Observations on Continuously Growing Roots of the Sloth and the K14-Eda
EVOLUTION & DEVELOPMENT 10:2, 187–195 (2008) Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns Mark Tummersà and Irma Thesleff1 Institute of Biotechnology, Viikki Biocenter, University of Helsinki, Finland ÃAuthor for correspondence (email: [email protected]) 1Current address and address at time of work for both authors: Institute of Biotechnology, P.O. Box 56, FIN-00014 University of Helsinki, Finland. SUMMARY Root development is traditionally associated that it acts as a functional stem cell niche. Similarly we show with the formation of Hertwig’s epithelial root sheath (HERS), that continuously growing roots represented by the sloth molar whose fragments give rise to the epithelial cell rests of and K14-Eda transgenic incisor maintain a cervical loop with a Malassez (ERM). The HERS is formed by depletion of the small core of stellate reticulum cells around the entire core of stellate reticulum cells, the putative stem cells, in the circumference of the tooth and do not form a HERS, and still cervical loop, leaving only a double layer of the basal give rise to ERM. We propose that HERS is not a necessary epithelium with limited growth capacity. The continuously structure to initiate root formation. Moreover, we conclude that growing incisor of the rodent is subdivided into a crown analog crown vs. root formation, i.e. the production of enamel vs. half on the labial side, with a cervical loop containing a large cementum, and the differentiation of the epithelial cells into core of stellate reticulum, and its progeny gives rise to enamel ameloblasts vs. -

The Mechanism: How Dental Resorptions Occur in Ameloblastoma
orthodontic insight The mechanism: how dental resorptions occur in ameloblastoma Giovana Gonçalves Martins1,2, Ingrid Araújo de Oliveira3,4, Alberto Consolaro2,5 DOI: https://doi.org/10.1590/2177-6709.24.4.021-032.oin Knife-edge or blunt root resorptions characterize ameloblastomas and are pathognomonic for this tumor, because they dif- ferentiate ameloblastomas from simple bone cysts, odontogenic keratocysts and nasopalatine duct cysts, which do not lead to resorption of involved teeth. Despite the very high frequency and importance of these characteristics for a differential diagnosis, a microscopic examination should also be conducted before defining the diagnosis and the treatment plan for these cases. This paper describes a six-step hypothesis to explain the mechanism by which ameloblastomas promote the characteristic root resorptions found in association with these benign epithelial tumors, which have a fibrous capsule formed by islands and epithelial cords that mimic the dental lamina, invade neighboring tissues and release mediators (IL-1, EGF) of tooth and root resorption. This hypothesis may be one more explanation for the tooth resorptions sometimes found in orthodontic records, and may help differentiate the root resorptions that are specific to the orthodontic practice. Keywords: Tooth resorption. Root resorption. Ameloblastoma. Lesions of the jaws. Orthodontics. As reabsorções radiculares “em plano” ou “em corte” são características do ameloblastoma e foram consideradas patogno- mônicas dessa lesão, diferenciando-o do cisto ósseo simples, queratocisto odontogênico e do cisto nasopalatino — nos quais elas estão ausentes, nos dentes envolvidos. Apesar dessa frequência elevadíssima e importante no diagnóstico diferencial, não se pode dispensar o exame microscópico, para um diagnóstico definitivo, no planejamento terapêutico do caso. -

Laser Ablation of Modern Human Cementum the Examination Of
Laser Ablation of Modern Human Cementum The examination of trace element profiles by Lisa Lefever A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfilment of the requirements of the degree of MASTER OF ARTS Department of Anthropology University of Manitoba Winnipeg, Manitoba ©2010 ii Abstract This study used LA-ICP-MS on a documented sample of modern teeth to sample from a continuous line across the cementum increments thus creating a temporal line graph of the elemental composition against distance. The knowledge of cementum was extended through (1) a more complete elemental composition analysis and (2) the relation of element distribution to the ultrastructure structure throughout the life of a tooth. This study was exploratory and demonstrated that lead, zinc, mercury, and barium follow the same general line of changes, and most likely represent changes in health and exposure to these metals in the general environment. Copper, manganese and vanadium varied very little. Technological limitations prevented the examination of element levels in any one annulation. iii Acknowledgements I would like to thank my advisor, Rob Hoppa, who constantly encouraged me to finish, and managed to wade through my random writings and massive literature review. I greatly appreciate the chance to show I can eventually make my way through a thesis. Secondly, I could not have accomplished any of this without the help from the rest of my external committee member, Dr Norman Halden, who graciously showed me the ways of laser ablation and trace elements, and granted me access to his cool toys.