Development of Immortalized Hertwig's Epithelial Root Sheath Cell
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
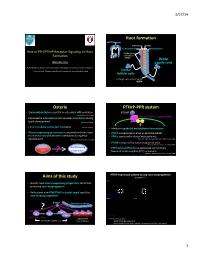
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Benign Cementoblastoma Associated with an Unerupted Third Molar - a Case Report
CORE Metadata, citation and similar papers at core.ac.uk Provided by Directory of Open Access Journals BENIGN CEMENTOBLASTOMA ASSOCIATED WITH AN UNERUPTED THIRD MOLAR - A CASE REPORT J.Dinakar* M.S.Senthil Kumar** Shiju Mathew Jacob*** *Professor & HOD, Department of Oral Pathology, ** Reader, *** Lecturer, Department of Oral Surgery, Sri Ramakrishna Dental College and Hospital, Coimbatore, Tamilnadu, India. ABSTRACT: Cementoblastoma is a rare odontogenic tumor derived from odontogenic ectomesenchyme of cementoblast origin that forms cementum layer on the roots of a tooth. A case report is presented of a patient treated with surgical excision of Cementoblastoma associated with an unerupted infected right lower third molar tooth. Key words: Cementoblastoma, Odontogenic tumour, unerupted third molar. The cell of origin is cementoblast. INTRODUCTION: Clinically it causes bony expansion. The commonest site is the posterior region of Cementoblastoma is an odontogenic the mandible. In the radiograph it is seen tumor of ectomesenchymal origin. It is as large radiopaque mass associated with also called cementoma. They are large the root of the tooth. We report a case of bulbous mass of cementum or cementum- Benign Cementoblastoma from Sri like tissue on roots of teeth. Ramakrishna Dental College & Hospital, Coimbatore. restriction in opening the mouth and intra oral examination reveals a partially CASE REPORT: erupted third molar tooth with pus discharge. A panoramic radiograph A 41 year old man presented to our showed a radio-opaque, dense, department with a complaint of pain and amorphous, irregularly shaped mass swelling in the right lower half of the face. measuring 2.2 x 1.5cm attached with the Patient gave history of intermittent pain third molar (Fig 1,1a). -

6 Development of the Teeth: Root and Supporting Structures Nagat M
AVERY Chap.06 27-11-2002 10:09 Pagina 108 108 II Development of the Teeth and Supporting Structures 6 Development of the Teeth: Root and Supporting Structures Nagat M. ElNesr and James K. Avery Chapter Outline Introduction Introduction... 108 Objectives... 108 Root development is initiated through the contributions Root Sheath Development... 109 of the cells originating from the enamel organ, dental Single-Root Formation... 110 papilla, and dental follicle. The cells of the outer enamel Multiple-Root Formation... 111 epithelium contact the inner enamel epithelium at the Root Formation Anomalies... 112 base of the enamel organ, the cervical loop (Figs. 6.1 and Fate of the Epithelial Root Sheath (Hertwig's Sheath)... 113 6.2A). Later, with crown completion, the cells of the cer- Dental Follicle... 114 vical loop continue to grow away from the crown and Development of (Intermediate) Cementum... 116 become root sheath cells (Figs. 6.2B and 6.3). The inner Cellular and Acellular Cementum... 116 root sheath cells cause root formation by inducing the Development of the Periodontal Ligament... 117 adjacent cells of the dental papilla to become odonto- Development of the Alveolar Process... 119 blasts, which in turn will form root dentin. The root Summary... 121 sheath will further dictate whether the tooth will have Self-Evaluation Review... 122 single or multiple roots. The remainder of the cells of the dental papilla will then become the cells of the root pulp.The third compo- nent in root formation, the dental follicle, is the tissue that surrounds the enamel organ, the dental papilla, and the root. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Oral Histology Dentinogenesis
Lecture 3 Oral Histology Dr. Lubna Dentinogenesis Dentin formation (Dentinogenesis): Begins at the bell stage development in the papillary tissue adjacent to the concave tip of the folded inner enamel epithelium the site where cuspal development begins. From that point, dentin formation spreads down the cusp slope as far as the cervical loop of the enamel organ, and the dentin thickens until all the coronal dentin is formed. In multicusped teeth, dentin formation begins independently at the sites of each future cusp tip and again spreads down the flanks of the cusp slopes until fusion with adjacent formative centers occurs. Dentin thus formed constitutes the dentin of the crown of the tooth, or coronal dentin. Odontoblasts Differentiation:- The differentiation odontoblasts from the dental papilla in normal development is brought about by the expression of signaling molecules and growth factors in the cells of the inner enamel epithelium illustrate the differentiation sequence, The few organelles. At this time they are separated from the inner enamel epithelium by cell free zone that contains some fine collagen fibrils. Almost immediately after cells of the inner enamel epithelium reverse polarity, changes also occur in the adjacent dental papilla. The ectomesenchymal cells adjoining this zone rapidly enlarge and elongate to become preodontoblasts first and then odontoblasts as their cytoplasm increases in volume to contain increasing amounts of protein-synthesizing organelles. Then this zone gradually is eliminated as the odontoblasts differentiate and increase in size and occupy this zone. These newly differentiated cells are characterized by being highly polarized (40 µm in length and 7 µm in width), with their nuclei positioned away from the inner enamel epithelium. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages
Downloaded from http://cshperspectives.cshlp.org/ on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages Maria Jussila and Irma Thesleff Developmental Biology Program Institute of Biotechnology, Biokeskus 1, P.O. Box 56, University of Helsinki, Helsinki FIN-00014, Finland Correspondence: maria.jussila@helsinki.fi SUMMARY Teeth develop as ectodermal appendages from epithelial and mesenchymal tissues. Tooth organogenesis is regulated by an intricate network of cell–cell signaling during all steps of development. The dental hard tissues, dentin, enamel, and cementum, are formed by unique cell types whose differentiation is intimately linked with morphogenesis. During evolution the capacity for tooth replacement has been reduced in mammals, whereas teeth have acquired more complex shapes. Mammalian teeth contain stem cells but they may not provide a source for bioengineering of human teeth. Therefore it is likely that nondental cells will have to be reprogrammed for the purpose of clinical tooth regeneration. Obviously this will require understanding of the mechanisms of normal development. The signaling networks mediating the epithelial-mesenchymal interactions during morphogenesis are well characterized but the molecular signatures of the odontogenic tissues remain to be uncovered. Outline 1 Morphogenesis and cell 4 Regulation of tooth replacement, continuous differentiation during tooth development growth, and stem cells in teeth 2 Signal networks and signaling centers 5 Future challenges: stem cell-based bioengineering of teeth 3 Regulation of the identity and 6 Concluding remarks differentiation of odontogenic mesenchymal and epithelial cell lineages References Editors: Patrick P.L. -

Effects of Lipid Metabolism on Mouse Incisor Dentinogenesis
www.nature.com/scientificreports OPEN Efects of lipid metabolism on mouse incisor dentinogenesis Yutaro Kurotaki1,2,3, Nobuhiro Sakai2,3*, Takuro Miyazaki4, Masahiro Hosonuma2,3,5, Yurie Sato2,3,6, Akiko Karakawa2,3, Masahiro Chatani2,3, Mie Myers1, Tetsuo Suzawa7, Takako Negishi-Koga2,3,8, Ryutaro Kamijo7, Akira Miyazaki4, Yasubumi Maruoka1 & Masamichi Takami2,3* Tooth formation can be afected by various factors, such as oral disease, drug administration, and systemic illness, as well as internal conditions including dentin formation. Dyslipidemia is an important lifestyle disease, though the relationship of aberrant lipid metabolism with tooth formation has not been clarifed. This study was performed to examine the efects of dyslipidemia on tooth formation and tooth development. Dyslipidemia was induced in mice by giving a high-fat diet (HFD) for 12 weeks. Additionally, LDL receptor-defcient (Ldlr−/−) strain mice were used to analyze the efects of dyslipidemia and lipid metabolism in greater detail. In the HFD-fed mice, incisor elongation was decreased and pulp was signifcantly narrowed, while histological fndings revealed disappearance of predentin. In Ldlr−/− mice fed regular chow, incisor elongation showed a decreasing trend and pulp a narrowing trend, while predentin changes were unclear. Serum lipid levels were increased in the HFD- fed wild-type (WT) mice, while Ldlr−/− mice given the HFD showed the greatest increase. These results show important efects of lipid metabolism, especially via the LDL receptor, on tooth homeostasis maintenance. In addition, they suggest a diferent mechanism for WT and Ldlr−/− mice, though the LDL receptor pathway may not be the only factor involved. A regular high-fat diet (HFD) has been shown to result in such lifestyle diseases as dyslipidemia, obesity, and diabetes1,2. -

Original Article BMP-2 Downregulation Is Involved in the Inhibition of Cementoblast Differentiation by Lithium
Int J Clin Exp Med 2016;9(2):823-830 www.ijcem.com /ISSN:1940-5901/IJCEM0017314 Original Article BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium Shang Gao1*, Xing-Fu Bao1*, Yu-Zhuo Wang1, Jing-Zheng Yi2, Min Hu1 1Department of Orthodontics, Stomatological Hospital of Jilin University, China; 2University of California in San Diego, USA. *Equal contributors. Received October 5, 2015; Accepted December 8, 2015; Epub February 15, 2016; Published February 29, 2016 Abstract: Cementum, which is formed by cementoblast, plays an important role in periodontal regeneration. Wnt signalling and BMP-2 are involved in the regulation of cementoblast differentiation, but little is known about the effect of lithium, an agonist of Wnt signalling, on cementoblast behaviour. In this research, OCCM-30 cementoblast was employed to investigate the influence of various concentrations of lithium on proliferation and differentiation. Cell metabolic assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, cell staining, alizarin red staining and alkaline phosphatase assay were performed. BMP-2 expression was also compared among different groups. Results showed no significant difference in proliferation, whereas differentiation was inhibited in the pres- ence of lithium. Given these results, along with previous reports, BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium. Keywords: Lithium, cementoblast, BMP-2 Introduction Bone morphogenetic protein 2 (BMP-2) is an autocrine and paracrine growth factor that is Cementum is a type of special mineralized tis- essential for the growth of mineralized tissue. sue covering tooth root that protects dental In osteoblasts, BMP-2 promotes the differentia- pulp from external stimulation. -

A Global Compendium of Oral Health
A Global Compendium of Oral Health A Global Compendium of Oral Health: Tooth Eruption and Hard Dental Tissue Anomalies Edited by Morenike Oluwatoyin Folayan A Global Compendium of Oral Health: Tooth Eruption and Hard Dental Tissue Anomalies Edited by Morenike Oluwatoyin Folayan This book first published 2019 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2019 by Morenike Oluwatoyin Folayan and contributors All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-3691-2 ISBN (13): 978-1-5275-3691-3 TABLE OF CONTENTS Foreword .................................................................................................. viii Introduction ................................................................................................. 1 Dental Development: Anthropological Perspectives ................................. 31 Temitope A. Esan and Lynne A. Schepartz Belarus ....................................................................................................... 48 Natallia Shakavets, Alexander Yatzuk, Klavdia Gorbacheva and Nadezhda Chernyavskaya Bangladesh ............................................................................................... -

Dental Manifestations in Bariatric Patients – Review of Literature
www.scielo.br/jaos Dental manifestations in bariatric patients – review of literature Carolina Silveira BARBOSA1, Gabriel Salles BARBÉRIO1, Vinicius Rizzo MARQUES1, Vitor de Oliveira BALDO1, Marília Afonso Rabelo BUZALAF2, Ana Carolina MAGALHÃES3 1- Undergraduate student, Bauru School of Dentistry, University of São Paulo. 2- DDS, MSc, PhD, Full Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo. 3- DDS, MSc, PhD, Assistant Professor, Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo. Corresponding address: Ana Carolina Magalhães - Faculdade de Odontologia de Bauru - Universidade de São Paulo - Departamento de Ciências Biológicas - Al. Octávio Pinheiro Brisolla, 9-75 Bauru-SP 17012-901 (Brazil) - Phone: + 55 14 32358246 Fax: + 55 14 32343164 - e-mail: [email protected] Received: August 19, 2009 - Accepted: February 19, 2010 ABSTRACT he rate of bariatric surgery has significantly risen in the past decade as an increasing Tprevalence of extreme obesity can be observed. Although bariatric surgery is an effective therapeutic modality for extreme obesity, it is associated with risk factors affecting also oral health. Based on an overview of the current literature, this paper presents a summary of dental manifestations in bariatric patients. Bariatric surgeries are associated with an increased risk for gastro-esophageal reflux which in turn might account for the higher amount of carious and erosive lesions observed in bariatric patients. As a result, also dentin hypersensitivity might be observed more frequently. The current data indicate that recommended postsurgical meal patterns and gastric reflux might increase the risk for dental lesions, particularly in the presence of other risk factors, such as consumption of sweet-tasting foods and acidic beverages.