Mesenchymal Stem Cells and Tooth Engineering Peng Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
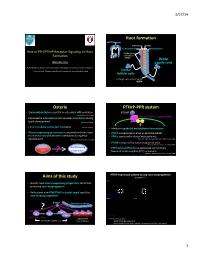
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Benign Cementoblastoma Associated with an Unerupted Third Molar - a Case Report
CORE Metadata, citation and similar papers at core.ac.uk Provided by Directory of Open Access Journals BENIGN CEMENTOBLASTOMA ASSOCIATED WITH AN UNERUPTED THIRD MOLAR - A CASE REPORT J.Dinakar* M.S.Senthil Kumar** Shiju Mathew Jacob*** *Professor & HOD, Department of Oral Pathology, ** Reader, *** Lecturer, Department of Oral Surgery, Sri Ramakrishna Dental College and Hospital, Coimbatore, Tamilnadu, India. ABSTRACT: Cementoblastoma is a rare odontogenic tumor derived from odontogenic ectomesenchyme of cementoblast origin that forms cementum layer on the roots of a tooth. A case report is presented of a patient treated with surgical excision of Cementoblastoma associated with an unerupted infected right lower third molar tooth. Key words: Cementoblastoma, Odontogenic tumour, unerupted third molar. The cell of origin is cementoblast. INTRODUCTION: Clinically it causes bony expansion. The commonest site is the posterior region of Cementoblastoma is an odontogenic the mandible. In the radiograph it is seen tumor of ectomesenchymal origin. It is as large radiopaque mass associated with also called cementoma. They are large the root of the tooth. We report a case of bulbous mass of cementum or cementum- Benign Cementoblastoma from Sri like tissue on roots of teeth. Ramakrishna Dental College & Hospital, Coimbatore. restriction in opening the mouth and intra oral examination reveals a partially CASE REPORT: erupted third molar tooth with pus discharge. A panoramic radiograph A 41 year old man presented to our showed a radio-opaque, dense, department with a complaint of pain and amorphous, irregularly shaped mass swelling in the right lower half of the face. measuring 2.2 x 1.5cm attached with the Patient gave history of intermittent pain third molar (Fig 1,1a). -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Gene Expression Profiles in Dental Follicles from Patients with Impacted
Odontology https://doi.org/10.1007/s10266-018-0342-9 ORIGINAL ARTICLE Gene expression profles in dental follicles from patients with impacted canines Pamela Uribe1 · Lena Larsson2 · Anna Westerlund1 · Maria Ransjö1 Received: 9 August 2017 / Accepted: 27 December 2017 © The Author(s) 2018. This article is an open access publication Abstract Animal studies suggest that the dental follicle (DF) plays a major role in tooth eruption. However, the role of the DF during tooth impaction and related root resorptions in adjacent teeth is not clear. The hypothesis for the present study is that expres- sion of regulatory factors involved in the bone remodelling process necessary for tooth eruption may difer between dental follicles from teeth with diferent clinical situations. We have analysed the gene expression profles in the DF obtained from impacted canines, with (N = 3) or without (N = 5) signs of root resorption, and from control teeth (normal erupting teeth, mesiodens) (N = 3). DF from 11 patients (mean age: 13 years) obtains at the time of surgical exposure of the tooth. Due to the surgical time point, all teeth were in a late developmental stage. Gene expression related to osteoblast activation/bone formation, osteoclast recruitment and activation was analysed by RTqPCR. Genes related to bone formation (RUNX2, OSX, ALP, OCN, CX43) were highly expressed in all the samples, but osteoclast recruitment/activation markers (OPG, RANKL, MCP-1, CSF-1) were negligible. No apparent patterns or signifcant diferences in gene expression were found between impacted canines, with or without signs of root resorption, or when compared to control teeth. Our results suggest the DF regulation of osteoclastic activity is limited in the late pre-emergent stage of tooth development, irrespective if the tooth is normally erupting or impacted. -

Periodontal and Dental Follicle Collagen in Tooth Eruption
SCIENTIFIC ARCHIVES OF DENTAL SCIENCES (ISSN: 2642-1623) Volume 4 Issue 1 January 2021 Review Article Periodontal and Dental Follicle Collagen in Tooth Eruption Norman Randall Thomas* Professor Emeritus, Faculty of Medicine and Dentistry, University of Alberta, Canada *Corresponding Author: Norman Randall Thomas, Professor Emeritus, Faculty of Medicine and Dentistry, University of Alberta, Canada. Received: September 18, 2020; Published: October 20, 2020 Abstract occlusal position in the oral cavity while passive eruption occurs by loss of epithelial attachment to expose the clinical crown. Rodent Review of the process and mechanism of tooth eruption defines active eruption as coronal migration of the tooth to the functional teeth are considered excellent analogs of eruption because they have examples of limited and continuous eruption in the molar teeth and incisors respectively. Root resection studies on rat incisors exhibit normal active eruption rates due to a ‘force’ in the retained prime mover of eruption. Impeded and unimpeded eruption rates were grossly retarded when a collagen crosslinking inhibitor periodontal ligament (PDL). Since all four walls of the tooth and bone remain patent it confirms that the periodontium alone is the lathyrogen 0.3% AAN (aminoacetonitrile) was added to the drinking water of young 45 - 50 gm rats. Using the Bryer 1957 method of measurement of eruption it appeared that low concentrations (0.01%) lathyrogen in the drinking water of adult rats did not intrusion and dilaceration of the reference molar and incisor decreases impeded eruption in the lathyritic condition giving a false have significant retardation of unimpeded eruption rates. Histological, radiological, bone and tooth marker studies indicate that impression of increased unimpeded eruption. -

Clinical Significance of Dental Anatomy, Histology, Physiology, and Occlusion
1 Clinical Significance of Dental Anatomy, Histology, Physiology, and Occlusion LEE W. BOUSHELL, JOHN R. STURDEVANT thorough understanding of the histology, physiology, and Incisors are essential for proper esthetics of the smile, facial soft occlusal interactions of the dentition and supporting tissues tissue contours (e.g., lip support), and speech (phonetics). is essential for the restorative dentist. Knowledge of the structuresA of teeth (enamel, dentin, cementum, and pulp) and Canines their relationships to each other and to the supporting structures Canines possess the longest roots of all teeth and are located at is necessary, especially when treating dental caries. The protective the corners of the dental arches. They function in the seizing, function of the tooth form is revealed by its impact on masticatory piercing, tearing, and cutting of food. From a proximal view, the muscle activity, the supporting tissues (osseous and mucosal), and crown also has a triangular shape, with a thick incisal ridge. The the pulp. Proper tooth form contributes to healthy supporting anatomic form of the crown and the length of the root make tissues. The contour and contact relationships of teeth with adjacent canine teeth strong, stable abutments for fixed or removable and opposing teeth are major determinants of muscle function in prostheses. Canines not only serve as important guides in occlusion, mastication, esthetics, speech, and protection. The relationships because of their anchorage and position in the dental arches, but of form to function are especially noteworthy when considering also play a crucial role (along with the incisors) in the esthetics of the shape of the dental arch, proximal contacts, occlusal contacts, the smile and lip support. -

Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages
Downloaded from http://cshperspectives.cshlp.org/ on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages Maria Jussila and Irma Thesleff Developmental Biology Program Institute of Biotechnology, Biokeskus 1, P.O. Box 56, University of Helsinki, Helsinki FIN-00014, Finland Correspondence: maria.jussila@helsinki.fi SUMMARY Teeth develop as ectodermal appendages from epithelial and mesenchymal tissues. Tooth organogenesis is regulated by an intricate network of cell–cell signaling during all steps of development. The dental hard tissues, dentin, enamel, and cementum, are formed by unique cell types whose differentiation is intimately linked with morphogenesis. During evolution the capacity for tooth replacement has been reduced in mammals, whereas teeth have acquired more complex shapes. Mammalian teeth contain stem cells but they may not provide a source for bioengineering of human teeth. Therefore it is likely that nondental cells will have to be reprogrammed for the purpose of clinical tooth regeneration. Obviously this will require understanding of the mechanisms of normal development. The signaling networks mediating the epithelial-mesenchymal interactions during morphogenesis are well characterized but the molecular signatures of the odontogenic tissues remain to be uncovered. Outline 1 Morphogenesis and cell 4 Regulation of tooth replacement, continuous differentiation during tooth development growth, and stem cells in teeth 2 Signal networks and signaling centers 5 Future challenges: stem cell-based bioengineering of teeth 3 Regulation of the identity and 6 Concluding remarks differentiation of odontogenic mesenchymal and epithelial cell lineages References Editors: Patrick P.L. -

Overexpression of Hypoxia-Inducible Factor 1 Alpha Improves Immunomodulation by Dental Mesenchymal Stem Cells Victor G
Martinez et al. Stem Cell Research & Therapy (2017) 8:208 DOI 10.1186/s13287-017-0659-2 RESEARCH Open Access Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells Victor G. Martinez1*, Imelda Ontoria-Oviedo2, Carolina P. Ricardo3, Sian E. Harding3, Rosa Sacedon4, Alberto Varas4, Agustin Zapata5, Pilar Sepulveda2 and Angeles Vicente4* Abstract Background: Human dental mesenchymal stem cells (MSCs) are considered as highly accessible and attractive MSCs for use in regenerative medicine, yet some of their features are not as well characterized as other MSCs. Hypoxia-preconditioning and hypoxia-inducible factor 1 (HIF-1) alpha overexpression significantly improves MSC therapeutics, but the mechanisms involved are not fully understood. In the present study, we characterize immunomodulatory properties of dental MSCs and determine changes in their ability to modulate adaptive and innate immune populations after HIF-1 alpha overexpression. Methods: Human dental MSCs were stably transduced with green fluorescent protein (GFP-MSCs) or GFP-HIF-1 alpha lentivirus vectors (HIF-MSCs). A hypoxic-like metabolic profile was confirmed by mitochondrial and glycolysis stress test. Capacity of HIF-MSCs to modulate T-cell activation, dendritic cell differentiation, monocyte migration, and polarizations towards macrophages and natural killer (NK) cell lytic activity was assessed by a number of functional assays in co-cultures. The expression of relevant factors were determined by polymerase chain reaction (PCR) analysis and enzyme-linked immunosorbent assay (ELISA). Results: While HIF-1 alpha overexpression did not modify the inhibition of T-cell activation by MSCs, HIF-MSCs impaired dendritic cell differentiation more efficiently. In addition, HIF-MSCs showed a tendency to induce higher attraction of monocytes, which differentiate into suppressor macrophages, and exhibited enhanced resistance to NK cell-mediated lysis, which supports the improved therapeutic capacity of HIF-MSCs. -

Sinking Our Teeth in Getting Dental Stem Cells to Clinics for Bone Regeneration
International Journal of Molecular Sciences Review Sinking Our Teeth in Getting Dental Stem Cells to Clinics for Bone Regeneration Sarah Hani Shoushrah , Janis Lisa Transfeld , Christian Horst Tonk, Dominik Büchner , Steffen Witzleben , Martin A. Sieber, Margit Schulze and Edda Tobiasch * Department of Natural Sciences, Bonn-Rhein-Sieg University of Applied Sciences, von-Liebig- Strasse. 20, 53359 Rheinbach, Germany; [email protected] (S.H.S.); [email protected] (J.L.T.); [email protected] (C.H.T.); [email protected] (D.B.); [email protected] (S.W.); [email protected] (M.A.S.); [email protected] (M.S.) * Correspondence: [email protected] Abstract: Dental stem cells have been isolated from the medical waste of various dental tissues. They have been characterized by numerous markers, which are evaluated herein and differentiated into multiple cell types. They can also be used to generate cell lines and iPSCs for long-term in vitro research. Methods for utilizing these stem cells including cellular systems such as organoids or cell sheets, cell-free systems such as exosomes, and scaffold-based approaches with and without drug release concepts are reported in this review and presented with new pictures for clarification. These in vitro applications can be deployed in disease modeling and subsequent pharmaceutical research and also pave the way for tissue regeneration. The main focus herein is on the potential of dental stem cells for hard tissue regeneration, especially bone, by evaluating their potential for osteogenesis Citation: Shoushrah, S.H.; Transfeld, and angiogenesis, and the regulation of these two processes by growth factors and environmental J.L.; Tonk, C.H.; Büchner, D.; stimulators. -

Original Article BMP-2 Downregulation Is Involved in the Inhibition of Cementoblast Differentiation by Lithium
Int J Clin Exp Med 2016;9(2):823-830 www.ijcem.com /ISSN:1940-5901/IJCEM0017314 Original Article BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium Shang Gao1*, Xing-Fu Bao1*, Yu-Zhuo Wang1, Jing-Zheng Yi2, Min Hu1 1Department of Orthodontics, Stomatological Hospital of Jilin University, China; 2University of California in San Diego, USA. *Equal contributors. Received October 5, 2015; Accepted December 8, 2015; Epub February 15, 2016; Published February 29, 2016 Abstract: Cementum, which is formed by cementoblast, plays an important role in periodontal regeneration. Wnt signalling and BMP-2 are involved in the regulation of cementoblast differentiation, but little is known about the effect of lithium, an agonist of Wnt signalling, on cementoblast behaviour. In this research, OCCM-30 cementoblast was employed to investigate the influence of various concentrations of lithium on proliferation and differentiation. Cell metabolic assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, cell staining, alizarin red staining and alkaline phosphatase assay were performed. BMP-2 expression was also compared among different groups. Results showed no significant difference in proliferation, whereas differentiation was inhibited in the pres- ence of lithium. Given these results, along with previous reports, BMP-2 downregulation is involved in the inhibition of cementoblast differentiation by lithium. Keywords: Lithium, cementoblast, BMP-2 Introduction Bone morphogenetic protein 2 (BMP-2) is an autocrine and paracrine growth factor that is Cementum is a type of special mineralized tis- essential for the growth of mineralized tissue. sue covering tooth root that protects dental In osteoblasts, BMP-2 promotes the differentia- pulp from external stimulation. -

The Development of the Permanent Teeth(
ro o 1Ppr4( SVsT' r&cr( -too c The Development of the Permanent Teeth( CARMEN M. NOLLA, B.S., D.D.S., M.S.* T. is important to every dentist treat- in the mouth of different children, the I ing children to have a good under - majority of the children exhibit some standing of the development of the den- pattern in the sequence of eruption tition. In order to widen one's think- (Klein and Cody) 9 (Lo and Moyers). 1-3 ing about the impingement of develop- However, a consideration of eruption ment on dental problems and perhaps alone makes one cognizant of only one improve one's clinical judgment, a com- phase of the development of the denti- prehensive study of the development of tion. A measure of calcification (matura- the teeth should be most helpful. tion) at different age-levels will provide In the study of child growth and de- a more precise index for determining velopment, it has been pointed out by dental age and will contribute to the various investigators that the develop- concept of the organism as a whole. ment of the dentition has a close cor- In 1939, Pinney2' completed a study relation to some other measures of of the development of the mandibular growth. In the Laboratory School of the teeth, in which he utilized a technic for University of Michigan, the nature of a serial study of radiographs of the same growth and development has been in- individual. It became apparent that a vestigated by serial examination of the similar study should be conducted in same children at yearly intervals, utiliz- order to obtain information about all of ing a set of objective measurements the teeth.