Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Direct Dental Restorations on Periodontium - Clinical and Radiological Study
Effects of direct dental restorations on periodontium - clinical and radiological study Luiza Ungureanu, Albertine Leon, Cristina Nuca, Corneliu Amariei, Doru Petrovici Constanta, Romania Summary The authors have performed a clinical study - 175 crown obturations of class II, II, V and cavities have been analyzed in 125 patients, following their impact on the marginal periodontium and a radi- ological study - consisting of the analysis of 108 proximal amalgam obturations and of their nega- tive effects on the profound periodontium. The results showed alarming percentages (over 80% in the clinical examination and 87% in the radiological examination of improper restorations, which generated periodontal alterations, from gingivitis to chronic marginal progressive periodontitis. The percentage of 59.26% obturations that triggered different degrees of osseous lysis imposed the need of knowing the negative effects of direct restorations on the periodontium and also the impor- tance of applying the specific preventive measures. Key words: dental anatomy, gingival embrasure contact area, under- and over sizing, cervical exten- sion, osseous lysis. Introduction the antagonist tooth can trigger enlargement of the contact point during functional movements. Dental restorations and periodontal health This allows interdental impact of foodstuff, with are closely related: periodontal health is needed devastating consequent effects on interproximal for the correct functioning of all restorations periodontal tissues. while the functional stimulation due to dental Marginal occlusal ridges must be placed restorations is essential for periodontal protection. above the proximal contact surface, and must be Coronal obturations with improper occlusal rounded and smooth so as to allow the access of modeling, oversized proximally or on the dental floss. -
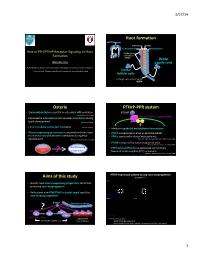
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Long-Term Uncontrolled Hereditary Gingival Fibromatosis: a Case Report
Long-term Uncontrolled Hereditary Gingival Fibromatosis: A Case Report Abstract Hereditary gingival fibromatosis (HGF) is a rare condition characterized by varying degrees of gingival hyperplasia. Gingival fibromatosis usually occurs as an isolated disorder or can be associated with a variety of other syndromes. A 33-year-old male patient who had a generalized severe gingival overgrowth covering two thirds of almost all maxillary and mandibular teeth is reported. A mucoperiosteal flap was performed using interdental and crevicular incisions to remove excess gingival tissues and an internal bevel incision to reflect flaps. The patient was treated 15 years ago in the same clinical facility using the same treatment strategy. There was no recurrence one year following the most recent surgery. Keywords: Gingival hyperplasia, hereditary gingival hyperplasia, HGF, hereditary disease, therapy, mucoperiostal flap Citation: S¸engün D, Hatipog˘lu H, Hatipog˘lu MG. Long-term Uncontrolled Hereditary Gingival Fibromatosis: A Case Report. J Contemp Dent Pract 2007 January;(8)1:090-096. © Seer Publishing 1 The Journal of Contemporary Dental Practice, Volume 8, No. 1, January 1, 2007 Introduction Hereditary gingival fibromatosis (HGF), also Ankara, Turkey with a complaint of recurrent known as elephantiasis gingiva, hereditary generalized gingival overgrowth. The patient gingival hyperplasia, idiopathic fibromatosis, had presented himself for examination at the and hypertrophied gingival, is a rare condition same clinic with the same complaint 15 years (1:750000)1 which can present as an isolated ago. At that time, he was treated with full-mouth disorder or more rarely as a syndrome periodontal surgery after the diagnosis of HGF component.2,3 This condition is characterized by had been made following clinical and histological a slow and progressive enlargement of both the examination (Figures 1 A-B). -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

Benign Cementoblastoma Associated with an Unerupted Third Molar - a Case Report
CORE Metadata, citation and similar papers at core.ac.uk Provided by Directory of Open Access Journals BENIGN CEMENTOBLASTOMA ASSOCIATED WITH AN UNERUPTED THIRD MOLAR - A CASE REPORT J.Dinakar* M.S.Senthil Kumar** Shiju Mathew Jacob*** *Professor & HOD, Department of Oral Pathology, ** Reader, *** Lecturer, Department of Oral Surgery, Sri Ramakrishna Dental College and Hospital, Coimbatore, Tamilnadu, India. ABSTRACT: Cementoblastoma is a rare odontogenic tumor derived from odontogenic ectomesenchyme of cementoblast origin that forms cementum layer on the roots of a tooth. A case report is presented of a patient treated with surgical excision of Cementoblastoma associated with an unerupted infected right lower third molar tooth. Key words: Cementoblastoma, Odontogenic tumour, unerupted third molar. The cell of origin is cementoblast. INTRODUCTION: Clinically it causes bony expansion. The commonest site is the posterior region of Cementoblastoma is an odontogenic the mandible. In the radiograph it is seen tumor of ectomesenchymal origin. It is as large radiopaque mass associated with also called cementoma. They are large the root of the tooth. We report a case of bulbous mass of cementum or cementum- Benign Cementoblastoma from Sri like tissue on roots of teeth. Ramakrishna Dental College & Hospital, Coimbatore. restriction in opening the mouth and intra oral examination reveals a partially CASE REPORT: erupted third molar tooth with pus discharge. A panoramic radiograph A 41 year old man presented to our showed a radio-opaque, dense, department with a complaint of pain and amorphous, irregularly shaped mass swelling in the right lower half of the face. measuring 2.2 x 1.5cm attached with the Patient gave history of intermittent pain third molar (Fig 1,1a). -

Intrusion of Incisors to Facilitate Restoration: the Impact on the Periodontium
Note: This is a sample Eoster. Your EPoster does not need to use the same format style. For example your title slide does not need to have the title of your EPoster in a box surrounded with a pink border. Intrusion of Incisors to Facilitate Restoration: The Impact on the Periodontium Names of Investigators Date Background and Purpose This 60 year old male had severe attrition of his maxillary and mandibular incisors due to a protrusive bruxing habit. The patient’s restorative dentist could not restore the mandibular incisors without significant crown lengthening. However, with orthodontic intrusion of the incisors, the restorative dentist was able to restore these teeth without further incisal edge reduction, crown lengthening, or endodontic treatment. When teeth are intruded in adults, what is the impact on the periodontium? The purpose of this study was to determine the effect of adult incisor intrusion on the alveolar bone level and on root length. Materials and Methods We collected the orthodontic records of 43 consecutively treated adult patients (aged > 19 years) from four orthodontic practices. This project was approved by the IRB at our university. Records were selected based upon the following criteria: • incisor intrusion attempted to create interocclusal space for restorative treatment or correction of excessive anterior overbite • pre- and posttreatment periapical and cephalometric radiographs were available • no incisor extraction or restorative procedures affecting the cementoenamel junction during the treatment period pretreatment pretreatment Materials and Methods We used cephalometric and periapical radiographs to measure incisor intrusion. The radiographs were imported and the digital images were analyzed with Image J, a public-domain Java image processing program developed at the US National Institutes of Health. -
Sensitive Teeth.Qxp
Sensitive teeth may be a warning of more serious problems Do You Have Sensitive Teeth? If you have a common problem called “sensitive teeth,” a sip of iced tea or a cup of hot cocoa, the sudden intake of cold air or pressure from your toothbrush may be painful. Sensitive teeth can be experienced at any age as a momentary slight twinge to long-term severe discomfort. It is important to consult your dentist because sensitive teeth may be an early warning sign of more serious dental problems. Understanding Tooth Structure. What Causes Sensitive Teeth? To better understand how sensitivity There can be many causes for sensitive develops, we need to consider the teeth. Cavities, fractured teeth, worn tooth composition of tooth structure. The crown- enamel, cracked teeth, exposed tooth root, the part of the tooth that is most visible- gum recession or periodontal disease may has a tough, protective jacket of enamel, be causing the problem. which is an extremely strong substance. Below the gum line, a layer of cementum Periodontal disease is an infection of the protects the tooth root. Underneath the gums and bone that support the teeth. If left enamel and cementum is dentin. untreated, it can progress until bone and other supporting tissues are destroyed. This Dentin is a part of the tooth that contains can leave the root surfaces of teeth exposed tiny tubes. When dentin loses its and may lead to tooth sensitivity. protective covering and is exposed, these small tubes permit heat, cold, Brushing incorrectly or too aggressively may certain types of foods or pressure to injure your gums and can also cause tooth stimulate nerves and cells inside of roots to be exposed. -

Lecture 2 – Bone
Oral Histology Summary Notes Enoch Ng Lecture 2 – Bone - Protection of brain, lungs, other internal organs - Structural support for heart, lungs, and marrow - Attachment sites for muscles - Mineral reservoir for calcium (99% of body’s) and phosphorous (85% of body’s) - Trap for dangerous minerals (ex:// lead) - Transduction of sound - Endocrine organ (osteocalcin regulates insulin signaling, glucose metabolism, and fat mass) Structure - Compact/Cortical o Diaphysis of long bone, “envelope” of cuboid bones (vertebrae) o 10% porosity, 70-80% calcified (4x mass of trabecular bone) o Protective, subject to bending/torsion/compressive forces o Has Haversian system structure - Trabecular/Cancellous o Metaphysis and epiphysis of long bone, cuboid bone o 3D branching lattice formed along areas of mechanical stress o 50-90% porosity, 15-25% calcified (1/4 mass of compact bone) o High surface area high cellular activity (has marrow) o Metabolic turnover 8x greater than cortical bone o Subject to compressive forces o Trabeculae lined with endosteum (contains osteoprogenitors, osteoblasts, osteoclasts) - Woven Bone o Immature/primitive, rapidly growing . Normally – embryos, newborns, fracture calluses, metaphyseal region of bone . Abnormally – tumors, osteogenesis imperfecta, Pagetic bone o Disorganized, no uniform orientation of collagen fibers, coarse fibers, cells randomly arranged, varying mineral content, isotropic mechanical behavior (behavior the same no matter direction of applied force) - Lamellar Bone o Mature bone, remodeling of woven -

Gene Expression Profiles in Dental Follicles from Patients with Impacted
Odontology https://doi.org/10.1007/s10266-018-0342-9 ORIGINAL ARTICLE Gene expression profles in dental follicles from patients with impacted canines Pamela Uribe1 · Lena Larsson2 · Anna Westerlund1 · Maria Ransjö1 Received: 9 August 2017 / Accepted: 27 December 2017 © The Author(s) 2018. This article is an open access publication Abstract Animal studies suggest that the dental follicle (DF) plays a major role in tooth eruption. However, the role of the DF during tooth impaction and related root resorptions in adjacent teeth is not clear. The hypothesis for the present study is that expres- sion of regulatory factors involved in the bone remodelling process necessary for tooth eruption may difer between dental follicles from teeth with diferent clinical situations. We have analysed the gene expression profles in the DF obtained from impacted canines, with (N = 3) or without (N = 5) signs of root resorption, and from control teeth (normal erupting teeth, mesiodens) (N = 3). DF from 11 patients (mean age: 13 years) obtains at the time of surgical exposure of the tooth. Due to the surgical time point, all teeth were in a late developmental stage. Gene expression related to osteoblast activation/bone formation, osteoclast recruitment and activation was analysed by RTqPCR. Genes related to bone formation (RUNX2, OSX, ALP, OCN, CX43) were highly expressed in all the samples, but osteoclast recruitment/activation markers (OPG, RANKL, MCP-1, CSF-1) were negligible. No apparent patterns or signifcant diferences in gene expression were found between impacted canines, with or without signs of root resorption, or when compared to control teeth. Our results suggest the DF regulation of osteoclastic activity is limited in the late pre-emergent stage of tooth development, irrespective if the tooth is normally erupting or impacted. -

The Cementum: Its Role in Periodontal Health and Disease*
THE JOURNAL OF PERIODONTOLOGY JULY, NINETEEN HUNDRED SIXTY ONE The Cementum: Its Role In Periodontal Health and Disease* by DONALD A. KERR, D.D.S., M.S.,** Ann Arbor, Michigan HE cementum is a specialized calcified tissue of mesenchymal origin which provides for the attachment of the periodontal fibers to the surface of the Troot. It consists of 45 to 50 per cent inorganic material and 50 to 55 per cent organic material with the inorganic material in a hydroxyl apatite structure. The primary cementum is formed initially by appositional growth from the dental sac and later from the periodontal membrane under the influence of cementoblasts. It is formed in laminated layers with the incorporation of Sharpey's fibers into a fibrillar matrix which undergoes calcification. Cementum deposition is a Continuous process throughout life with new cementum being deposited over the old cemental surface. Cementum is formed by the organiza• tion of collagen fibrils which are cemented together by a matrix produced by the polymerization of mucopolysaccharides. This material is designated as cementoid and becomes mature cementum upon calcification. The significance of the continuous deposition of cementum has received various interpretations. 1. Continuous deposition of cementum is necessary for the reattachment of periodontal fibers which have been destroyed or which require reorientation due to change in position of teeth. It is logical that there should be a continuous deposition of cementum because it is doubtful that the initial fibers are retained throughout the life of the tooth, and therefore new fibers must be continually formed and attached by new cementum. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b.