Identification of Arenin, a Novel Kunitz-Like Polypeptide from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes Chrysoscelis
University of Dayton eCommons Honors Theses University Honors Program 4-1-2019 Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Maria P. LaBello University of Dayton Follow this and additional works at: https://ecommons.udayton.edu/uhp_theses Part of the Biology Commons eCommons Citation LaBello, Maria P., "Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis" (2019). Honors Theses. 218. https://ecommons.udayton.edu/uhp_theses/218 This Honors Thesis is brought to you for free and open access by the University Honors Program at eCommons. It has been accepted for inclusion in Honors Theses by an authorized administrator of eCommons. For more information, please contact [email protected], [email protected]. Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in Erythrocytes from the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Page | i Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Abstract Dryophytes chrysoscelis, commonly known as Cope’s gray treefrog, is a freeze tolerant anuran that freezes up to 65% of extracellular fluid during winter to survive. Glycerol is presumably used as a cryoprotectant during a period of cold-acclimation to protect cells from permanent damage due to hypoosmotic stress upon freezing and thawing. The passage of glycerol and water during cold-acclimation is mediated through aquaglyceroporin HC-3 in the nucleated erythrocytes (RBCs) of D. -

Phylogeography of the American Green Treefrog Species Group
PHYLOGEOGRAPHY OF THE AMERICAN GREEN TREEFROG SPECIES GROUP by PAUL NATHANIEL PASICHNYK Presented to the Faculty of the Graduate School of The University of Texas at Arlington In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON November 2016 Copyright © by Paul N. Pasichnyk 2016 All Rights Reserved ii Acknowledgments A student’s graduate work is definitely not something that is done alone. I have received help from numerous people over the years and without their contributions, this would not have been possible. First, I would like to thank my parents for their tremendous support and motivation as I grew up and provided me with a foundation and love in education. Secondly, I would like to thank the person who has had to help me daily with tasks as simple as a pat on the back to as complex as reading a rough draft of a dissertation that might as well be in a foreign language, my wife Karen. My in-laws deserve special thanks as well, I do not believe that it is possible for others’ in-laws to be as supportive and helpful as mine. Then I would like to thank all the undergrads and friends who have helped see this to completion: Richard Hanna, Sari Mahon, Sarah Young, Nicole Lopez, Annamarie Slaven, Leslie Segovia, Pankaj BC, Matt Moseley, Christian Cox, Jeff Streicher, Claudia Marquez, Jesse Meik, Walter Schargel, Corey Roelke, Matt Watson, Rebbekah Watson, Thomas Eimermacher, Utpal Smart, David Sanchez, Jacobo Reyes-Velasco, Melissa Muenzler, Carl Franklin, Linda Taylor, Gloria Burlingham, Sherri Echols, and Paulette Williams. -

Phylogeography Reveals an Ancient Cryptic Radiation in East-Asian Tree
Dufresnes et al. BMC Evolutionary Biology (2016) 16:253 DOI 10.1186/s12862-016-0814-x RESEARCH ARTICLE Open Access Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages Christophe Dufresnes1, Spartak N. Litvinchuk2, Amaël Borzée3,4, Yikweon Jang4, Jia-Tang Li5, Ikuo Miura6, Nicolas Perrin1 and Matthias Stöck7,8* Abstract Background: In contrast to the Western Palearctic and Nearctic biogeographic regions, the phylogeography of Eastern-Palearctic terrestrial vertebrates has received relatively little attention. In East Asia, tectonic events, along with Pleistocene climatic conditions, likely affected species distribution and diversity, especially through their impact on sea levels and the consequent opening and closing of land-bridges between Eurasia and the Japanese Archipelago. To better understand these effects, we sequenced mitochondrial and nuclear markers to determine phylogeographic patterns in East-Asian tree frogs, with a particular focus on the widespread H. japonica. Results: We document several cryptic lineages within the currently recognized H. japonica populations, including two main clades of Late Miocene divergence (~5 Mya). One occurs on the northeastern Japanese Archipelago (Honshu and Hokkaido) and the Russian Far-East islands (Kunashir and Sakhalin), and the second one inhabits the remaining range, comprising southwestern Japan, the Korean Peninsula, Transiberian China, Russia and Mongolia. Each clade further features strong allopatric Plio-Pleistocene subdivisions (~2-3 Mya), especially among continental and southwestern Japanese tree frog populations. Combined with paleo-climate-based distribution models, the molecular data allowed the identification of Pleistocene glacial refugia and continental routes of postglacial recolonization. Phylogenetic reconstructions further supported genetic homogeneity between the Korean H. -

2021Harcourtajmres
Bangor University MASTERS BY RESEARCH Interspecific Differences in Treefrog Response to Artificial Light at Night and Spectral Manipulation Harcourt, Alexander Award date: 2021 Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 Interspecific Differences in Treefrog Response to Artificial Light at Night and Spectral Manipulation Understanding the effect of artificial light at night (ALAN) on biodiversity is a key research topic of the 21st Century. Evidence suggests that LED lighting may be particularly disruptive due to strong short-wavelength emissions. Spectral manipulation of LED lighting to reduce these emissions may mitigate some disturbance, although further research is required to assess its value in comparison with other techniques. The impact of LED lighting has been documented for many species, however, amphibians remain relatively under-studied. Amphibians may be particularly sensitive to the effects of ALAN due to specialised vision adapted for low-light environments and reduced mobility. -
Phylogenetic Relationship Among Hylidae and Mitochondrial Protein-Coding Gene Expression in Response to Freezing and Ano
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/332083499 The complete mitochondrial genome of Dryophytes versicolor: Phylogenetic relationship among Hylidae and mitochondrial protein-coding gene expression in response to freezing and ano... Article in International Journal of Biological Macromolecules · March 2019 DOI: 10.1016/j.ijbiomac.2019.03.220 CITATIONS READS 5 209 6 authors, including: Jia-Yong Zhang Bryan E Luu Zhejiang Normal University McGill University 88 PUBLICATIONS 696 CITATIONS 29 PUBLICATIONS 193 CITATIONS SEE PROFILE SEE PROFILE Danna Yu Leping Zhang Zhejiang Normal University Westlake University 57 PUBLICATIONS 255 CITATIONS 18 PUBLICATIONS 104 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Hibernation Metabolomics View project New project: 1)The frog mitochondrial genome project: evolution of frog mitochondrial genomes and their gene expression View project All content following this page was uploaded by Jia-Yong Zhang on 08 April 2019. The user has requested enhancement of the downloaded file. International Journal of Biological Macromolecules 132 (2019) 461–469 Contents lists available at ScienceDirect International Journal of Biological Macromolecules journal homepage: http://www.elsevier.com/locate/ijbiomac The complete mitochondrial genome of Dryophytes versicolor: Phylogenetic relationship among Hylidae and mitochondrial protein-coding gene expression in response to freezing and -

Translocation of an Endangered Endemic Korean Treefrog Dryophytes Suweonensis
A. Borzée, Y.-I. Kim, Y.-E. Kim & Y. Jang / Conservation Evidence (2018) 15, 6-11 Translocation of an endangered endemic Korean treefrog Dryophytes suweonensis Amaël Borzée1,2, Ye Inn Kim2, Ye Eun Kim2* & Yikweon Jang2,3*. 1 Laboratory of Behavioral Ecology and Evolution, School of Biological Sciences, Seoul National University, Seoul, 08826, Republic of Korea 2 Department of Life Sciences and Division of EcoScience, Ewha Womans University, Seoul, 03760, Republic of Korea 3 Interdisciplinary Program of EcoCreative, Ewha Womans University, Seoul, 03760, Republic of Korea SUMMARY Endangered species in heavily modified landscapes may be vulnerable to extinction if no conservation plan is implemented. The Suweon treefrog Dryophytes suweonensis is an endemic endangered species from the Korean Peninsula. In an attempt to conserve the species, a translocation plan was implemented in the city of Suwon. The receptor site was a specially modified island in a reservoir. Egg clutches were collected from four nearby sites, and were hatched and reared in a laboratory during 2015. One hundred and fifty froglets were released at the new site. In 2016, one year after the translocation, calling male D. suweonensis, and newly hatched tadpoles and juveniles were recorded. Juveniles were seen until the last week before hibernation in autumn 2016. However, only a single male was recorded calling in 2017. The population was consequently considered functionally extinct. Failure of the translocation most likely arose from mismanagement of the vegetation surrounding the wetlands, and the resulting inability of the site to fulfil the ecological requirements of the species. The project allowed the development of rearing protocols for the species, and defined its ecological requirements. -

Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae)
Zootaxa 4104 (1): 001–109 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4104.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:D598E724-C9E4-4BBA-B25D-511300A47B1D ZOOTAXA 4104 Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae) WILLIAM E. DUELLMAN1,3, ANGELA B. MARION2 & S. BLAIR HEDGES2 1Biodiversity Institute, University of Kansas, 1345 Jayhawk Blvd., Lawrence, Kansas 66045-7593, USA 2Center for Biodiversity, Temple University, 1925 N 12th Street, Philadelphia, Pennsylvania 19122-1601, USA 3Corresponding author. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by M. Vences: 27 Oct. 2015; published: 19 Apr. 2016 WILLIAM E. DUELLMAN, ANGELA B. MARION & S. BLAIR HEDGES Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae) (Zootaxa 4104) 109 pp.; 30 cm. 19 April 2016 ISBN 978-1-77557-937-3 (paperback) ISBN 978-1-77557-938-0 (Online edition) FIRST PUBLISHED IN 2016 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/j/zt © 2016 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. -

1 Freeze Tolerance and Cryoprotection Of
FREEZE TOLERANCE AND CRYOPROTECTION OF ERYTHROCYTES FROM DRYOPHYTES CHRYSOSCELIS Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Loren Geiss Dayton, Ohio August 2018 1 FREEZE TOLERANCE AND CRYOPROTECTION OF ERYTHROCYTES FROM DRYOPHYTES CHRYSOSCELIS Name: Geiss, Loren Vanessa APPROVED BY: ________________________ Carissa M. Krane, Ph.D. Professor, Dept. of Biology, University of Dayton Faculty Advisor ________________________ Amit Singh, Ph.D. Associate Professor, Dept. of Biology, University of Dayton Committee Member ________________________ Yvonne Sun, Ph.D. Assistant Professor, Dept. of Biology, University of Dayton Committee Member ________________________ David L. Goldstein, Ph.D. Professor, Dept. of Biological Sciences, Wright State University Committee Member ii ABSTRACT FREEZE TOLERANCE AND CRYOPROTECTION OF ERYTHROCYTES FROM DRYOPHYTES CHRYSOSCELIS Name: Geiss, Loren Vanessa University of Dayton Advisor: Dr. Carissa M. Krane Dryophytes chrysoscelis (Cope’s gray treefrog) is a freeze-tolerant anuran that survives the freezing of its extracellular fluids. Treefrogs accumulate glycerol and urea in the plasma during cold acclimation and at the onset of freezing. It is hypothesized that glycerol and urea function as cryoprotectants by minimizing osmotically induced cell damage during freezing and thawing; and therefore, the post-freeze viability of red blood cells (RBCs) would improve when frozen in phosphate buffered saline (PBS) containing glycerol or urea. In the present study, erythrocytes were obtained from warm (22°C) and cold-acclimated (4°C) frogs and suspended in PBS. RBCs were frozen in PBS that ranged in osmolarity: PBS at 230 mM is hypoosmotic/hypotonic, 280 mM PBS is isosmotic/isotonic, and PBS was made hyperosmotic/hypertonic by the addition of 150 mM solutes. -

Distribution and Genetic Diversity of the Amphibian Chytrid in Japan
Journal of Fungi Article Distribution and Genetic Diversity of the Amphibian Chytrid in Japan Koichi Goka 1,*, Jun Yokoyama 2 and Atsushi Tominaga 3 1 National Institute for Environmental Studies, 16-2 Onogawa, Tsukuba 305-8506, Japan 2 Department of Biology, Faculty of Sciences, Yamagata University, 1-4-12 Kojirakawa, Yamagata-shi, Yamagata 990-8560, Japan; [email protected] 3 Department of Natural Sciences, Faculty of Education, University of the Ryukyus, 1 Senbaru, Nishihara, Okinawa 901-0213, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-29-850-2480; Fax: +81-29-850-2582 Abstract: While research on frog chytrid fungus Batrachochytrium dendrobatidis (Bd), an infectious disease that threatens amphibian diversity, continues to advance worldwide, little progress has been made in Japan since around 2010. The reason for this is, which we pointed out in 2009, that the origin of frog chytrid fungus may be in the East Asian region, including Japan based on the Bd ITS-DNA variation, and as few cases of mass mortality caused by this fungus have been observed in wild amphibian populations in Japan, the interest of the Japanese government and the general public in Bd has waned. However, we believe that organizing the data obtained so far in Japan and distributing the status of frog chytrid fungus in Japan to the world will provide useful insight for future risk management of this pathogen. We collected more than 5500 swab samples from wild amphibians throughout Japan from 2009 to 2010. Then, we investigated the infection status using the Nested-PCR method. -

Captive Wildlife Regulations, 2021, W-13.12 Reg 5
1 CAPTIVE WILDLIFE, 2021 W-13.12 REG 5 The Captive Wildlife Regulations, 2021 being Chapter W-13.12 Reg 5 (effective June 1, 2021). NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 W-13.12 REG 5 CAPTIVE WILDLIFE, 2021 Table of Contents PART 1 PART 5 Preliminary Matters Zoo Licences and Travelling Zoo Licences 1 Title 38 Definition for Part 2 Definitions and interpretation 39 CAZA standards 3 Application 40 Requirements – zoo licence or travelling zoo licence PART 2 41 Breeding and release Designations, Prohibitions and Licences PART 6 4 Captive wildlife – designations Wildlife Rehabilitation Licences 5 Prohibition – holding unlisted species in captivity 42 Definitions for Part 6 Prohibition – holding restricted species in captivity 43 Standards for wildlife rehabilitation 7 Captive wildlife licences 44 No property acquired in wildlife held for 8 Licence not required rehabilitation 9 Application for captive wildlife licence 45 Requirements – wildlife rehabilitation licence 10 Renewal 46 Restrictions – wildlife not to be rehabilitated 11 Issuance or renewal of licence on terms and conditions 47 Wildlife rehabilitation practices 12 Licence or renewal term PART 7 Scientific Research Licences 13 Amendment, suspension, -
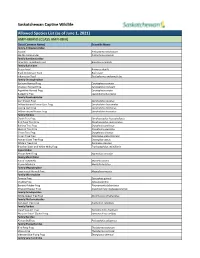
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Universidad Nacional Autónoma De México
UNIVERSIDAD NACIONAL AUTÓNOMA DE MÉXICO FACULTAD DE CIENCIAS Bases genéticas para la diferenciación de Dryophytes arboricola de D. eximius (Anura: Hylidae), evidencia mas allá del canto T E S I S QUE PARA OBTENER EL TÍTULO DE: BIÓLOGA P R E S E N T A : PAULINA FERNÁNDEZ SÁNCHEZ DIRECTOR DE TESIS: Dr. VÍCTOR HUGO REYNOSO ROSALES CIUDAD UNIVERSITARIA, CIUDAD DE MÉXICO, 2018 UNAM – Dirección General de Bibliotecas Tesis Digitales Restricciones de uso DERECHOS RESERVADOS © PROHIBIDA SU REPRODUCCIÓN TOTAL O PARCIAL Todo el material contenido en esta tesis esta protegido por la Ley Federal del Derecho de Autor (LFDA) de los Estados Unidos Mexicanos (México). El uso de imágenes, fragmentos de videos, y demás material que sea objeto de protección de los derechos de autor, será exclusivamente para fines educativos e informativos y deberá citar la fuente donde la obtuvo mencionando el autor o autores. Cualquier uso distinto como el lucro, reproducción, edición o modificación, será perseguido y sancionado por el respectivo titular de los Derechos de Autor. 1. Datos del alumno Fernández Sánchez Paulina Universidad Nacional Autónoma de México México Facultad de Ciencias Biología 309040314 2. Datos del tutor Dr. Víctor Hugo Reynoso Rosales 3. Datos sinodal 1 Dr. Marco Alejandro Suárez Atilano 4. Datos sinodal 2 M. en C. Enrique Scheinvar Gottdiener 5. Datos sinodal 3 M. en C. Andrea Rubí Jiménez Marín 6. Datos sinodal 4 M. en C. Ricardo Canek Rivera Arroyo 7. Datos del trabajo escrito Bases genéticas para la diferenciación de Dryophytes arboricola de D. eximius (Anura Hylidae), evidencia mas allá del canto. 72 páginas 2018 AGRADECIMIENTOS Al Dr.