L -Asparagine Monohydrate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amino Acid Recognition by Aminoacyl-Trna Synthetases
www.nature.com/scientificreports OPEN The structural basis of the genetic code: amino acid recognition by aminoacyl‑tRNA synthetases Florian Kaiser1,2,4*, Sarah Krautwurst3,4, Sebastian Salentin1, V. Joachim Haupt1,2, Christoph Leberecht3, Sebastian Bittrich3, Dirk Labudde3 & Michael Schroeder1 Storage and directed transfer of information is the key requirement for the development of life. Yet any information stored on our genes is useless without its correct interpretation. The genetic code defnes the rule set to decode this information. Aminoacyl-tRNA synthetases are at the heart of this process. We extensively characterize how these enzymes distinguish all natural amino acids based on the computational analysis of crystallographic structure data. The results of this meta-analysis show that the correct read-out of genetic information is a delicate interplay between the composition of the binding site, non-covalent interactions, error correction mechanisms, and steric efects. One of the most profound open questions in biology is how the genetic code was established. While proteins are encoded by nucleic acid blueprints, decoding this information in turn requires proteins. Te emergence of this self-referencing system poses a chicken-or-egg dilemma and its origin is still heavily debated 1,2. Aminoacyl-tRNA synthetases (aaRSs) implement the correct assignment of amino acids to their codons and are thus inherently connected to the emergence of genetic coding. Tese enzymes link tRNA molecules with their amino acid cargo and are consequently vital for protein biosynthesis. Beside the correct recognition of tRNA features3, highly specifc non-covalent interactions in the binding sites of aaRSs are required to correctly detect the designated amino acid4–7 and to prevent errors in biosynthesis5,8. -

Asparagine-Proline Sequence Within Membrane-Spanning Segment of SREBP Triggers Intramembrane Cleavage by Site-2 Protease
Asparagine-proline sequence within membrane-spanning segment of SREBP triggers intramembrane cleavage by Site-2 protease Jin Ye*†, Utpal P. Dave´ *†, Nick V. Grishin‡, Joseph L. Goldstein*§, and Michael S. Brown*§ Departments of *Molecular Genetics and ‡Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9046 Contributed by Joseph L. Goldstein, March 16, 2000 The NH2-terminal domains of membrane-bound sterol regulatory nus. It translocates to the nucleus, where it activates more than element-binding proteins (SREBPs) are released into the cytosol by 20 genes encoding enzymes of cholesterol and fatty acid synthesis regulated intramembrane proteolysis, after which they enter the as well as the low density lipoprotein receptor (6, 7). When nucleus to activate genes encoding lipid biosynthetic enzymes. sterols build up in cells, the SREBP͞SCAP complex fails to exit Intramembrane proteolysis is catalyzed by Site-2 protease (S2P), a the ER, and it never reaches S1P (8, 9). As a result, the hydrophobic zinc metalloprotease that cleaves SREBPs at a mem- NH2-terminal domains of the SREBPs are no longer released brane-embedded leucine-cysteine bond. In the current study, we into the nucleus, and transcription of the target genes declines. use domain-swapping methods to localize the residues within This mechanism allows cholesterol to inhibit its own synthesis the SREBP-2 membrane-spanning segment that are required for and uptake, thereby preventing cholesterol overaccumulation in cleavage by S2P. The studies reveal a requirement for an asparag- cells. ine-proline sequence in the middle third of the transmembrane The human gene encoding S2P was cloned by complementa- segment. -

Amino Acid Chemistry
Handout 4 Amino Acid and Protein Chemistry ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES Amino Acid Chemistry I. Chemistry of amino acids A. General amino acid structure + HN3- 1. All amino acids are carboxylic acids, i.e., they have a –COOH group at the #1 carbon. 2. All amino acids contain an amino group at the #2 carbon (may amino acids have a second amino group). 3. All amino acids are zwitterions – they contain both positive and negative charges at physiological pH. II. Essential and nonessential amino acids A. Nonessential amino acids: can make the carbon skeleton 1. From glycolysis. 2. From the TCA cycle. B. Nonessential if it can be made from an essential amino acid. 1. Amino acid "sparing". 2. May still be essential under some conditions. C. Essential amino acids 1. Branched chain amino acids (isoleucine, leucine and valine) 2. Lysine 3. Methionine 4. Phenyalanine 5. Threonine 6. Tryptophan 1 Handout 4 Amino Acid and Protein Chemistry D. Essential during rapid growth or for optimal health 1. Arginine 2. Histidine E. Nonessential amino acids 1. Alanine (from pyruvate) 2. Aspartate, asparagine (from oxaloacetate) 3. Cysteine (from serine and methionine) 4. Glutamate, glutamine (from α-ketoglutarate) 5. Glycine (from serine) 6. Proline (from glutamate) 7. Serine (from 3-phosphoglycerate) 8. Tyrosine (from phenylalanine) E. Nonessential and not required for protein synthesis 1. Hydroxyproline (made postranslationally from proline) 2. Hydroxylysine (made postranslationally from lysine) III. Acidic, basic, polar, and hydrophobic amino acids A. Acidic amino acids: amino acids that can donate a hydrogen ion (proton) and thereby decrease pH in an aqueous solution 1. -

Nucleotide Base Coding and Am1ino Acid Replacemients in Proteins* by Emil L
VOL. 48, 1962 BIOCHEMISTRY: E. L. SAIITH 677 18 Britten, R. J., and R. B. Roberts, Science, 131, 32 (1960). '9 Crestfield, A. M., K. C. Smith, and F. WV. Allen, J. Biol. Chem., 216, 185 (1955). 20 Gamow, G., Nature, 173, 318 (1954). 21 Brenner, S., these PROCEEDINGS, 43, 687 (1957). 22 Nirenberg, M. WV., J. H. Matthaei, and 0. WV. Jones, unpublished data. 23 Crick, F. H. C., L. Barnett, S. Brenner, and R. J. Watts-Tobin, Nature, 192, 1227 (1961). 24 Levene, P. A., and R. S. Tipson, J. Biol. Ch-nn., 111, 313 (1935). 25 Gierer, A., and K. W. Mundry, Nature, 182, 1437 (1958). 2' Tsugita, A., and H. Fraenkel-Conrat, J. Mllot. Biol., in press. 27 Tsugita, A., and H. Fraenkel-Conrat, personal communication. 28 Wittmann, H. G., Naturwissenschaften, 48, 729 (1961). 29 Freese, E., in Structure and Function of Genetic Elements, Brookhaven Symposia in Biology, no. 12 (1959), p. 63. NUCLEOTIDE BASE CODING AND AM1INO ACID REPLACEMIENTS IN PROTEINS* BY EMIL L. SMITHt LABORATORY FOR STUDY OF HEREDITARY AND METABOLIC DISORDERS AND THE DEPARTMENTS OF BIOLOGICAL CHEMISTRY AND MEDICINE, UNIVERSITY OF UTAH COLLEGE OF MEDICINE Communicated by Severo Ochoa, February 14, 1962 The problem of which bases of messenger or template RNA' specify the coding of amino acids in proteins has been largely elucidated by the use of synthetic polyri- bonucleotides.2-7 For these triplet nucleotide compositions (Table 1), it is of in- terest to examine some of the presently known cases of amino acid substitutions in polypeptides or proteins of known structure. -

An Integrated Meta-Analysis of Peripheral Blood Metabolites and Biological Functions in Major Depressive Disorder
Molecular Psychiatry https://doi.org/10.1038/s41380-020-0645-4 ARTICLE An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder 1,2,3 1,2,3 1,2,3 1,3 1,3 4,5 1,3 1,3 Juncai Pu ● Yiyun Liu ● Hanping Zhang ● Lu Tian ● Siwen Gui ● Yue Yu ● Xiang Chen ● Yue Chen ● 1,2,3 1,3 1,3 1,3 1,3 1,2,3 Lining Yang ● Yanqin Ran ● Xiaogang Zhong ● Shaohua Xu ● Xuemian Song ● Lanxiang Liu ● 1,2,3 1,3 1,2,3 Peng Zheng ● Haiyang Wang ● Peng Xie Received: 3 June 2019 / Revised: 24 December 2019 / Accepted: 10 January 2020 © The Author(s) 2020. This article is published with open access Abstract Major depressive disorder (MDD) is a serious mental illness, characterized by high morbidity, which has increased in recent decades. However, the molecular mechanisms underlying MDD remain unclear. Previous studies have identified altered metabolic profiles in peripheral tissues associated with MDD. Using curated metabolic characterization data from a large sample of MDD patients, we meta-analyzed the results of metabolites in peripheral blood. Pathway and network analyses were then performed to elucidate the biological themes within these altered metabolites. We identified 23 differentially 1234567890();,: 1234567890();,: expressed metabolites between MDD patients and controls from 46 studies. MDD patients were characterized by higher levels of asymmetric dimethylarginine, tyramine, 2-hydroxybutyric acid, phosphatidylcholine (32:1), and taurochenode- soxycholic acid and lower levels of L-acetylcarnitine, creatinine, L-asparagine, L-glutamine, linoleic acid, pyruvic acid, palmitoleic acid, L-serine, oleic acid, myo-inositol, dodecanoic acid, L-methionine, hypoxanthine, palmitic acid, L-tryptophan, kynurenic acid, taurine, and 25-hydroxyvitamin D compared with controls. -
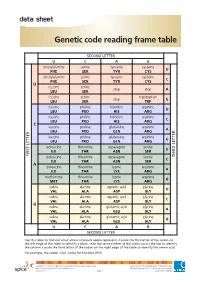
Genetic Code Reading Frame Table
data sheet Genetic code reading frame table SECOND LETTER U C A G phenylalanine serine tyrosine cysteine U PHE SER TYR CYS phenylalanine serine tyrosine cysteine C PHE SER TYR CYS U leucine serine stop stop A LEU SER leucine serine tryptophan stop G LEU SER TRP leucine proline histidine arginine U LEU PRO HIS ARG leucine proline histidine arginine C LEU PRO HIS ARG C leucine proline glutamine arginine A LEU PRO GLN ARG leucine proline glutamine arginine G LEU PRO GLN ARG isoleucine threonine asparagine serine U ILE THR ASN SER FIRST LETTER isoleucine threonine asparagine serine LETTERTHIRD C ILE THR ASN SER A isoleucine threonine lysine arginine A ILE THR LYS ARG methionine threonine lysine arginine G MET THR LYS ARG valine alanine aspartic acid glycine U VAL ALA ASP GLY valine alanine aspartic acid glycine C VAL ALA ASP GLY G valine alanine glutamic acid glycine A VAL ALA GLU GLY valine alanine glutamic acid glycine G VAL ALA GLU GLY U C A G SECOND LETTER Use this table to find out what amino acid each codon represents. Locate the first letter of the codon on the left edge of the table to identify a block, then the second letter of the codon across the top to identify the column. Locate the third letter of the codon on the right edge of the table to identify the amino acid. For example, the codon ‘CAU’ codes for histidine (HIS). ast0812 | Proteins 3: Genetic code reading frame table (data sheet) developed for the Department of Education WA © The University of Western Australia 2012 for conditions of use see spice.wa.edu.au/usage version 1.0 page 1 Licensed for NEALS. -

Analysis of Amino Acids by HPLC
Analysis of Amino Acids by HPLC Rita Steed Agilent Technologies, Inc. 800-227-9770 opt 3/opt3/opt 2 Amino Acid Analysis - Agilent Restricted Page 1 June 24, 2010 Outline • Amino Acids – Structure, Chemistry • Separation Considerations • Challenges • Instrumentation • Derivatization – OPA, FMOC • Overview of Separations • Examples Amino Acid Analysis - Agilent Restricted Page 2 June 24, 2010 Amino Acids – Structure, Chemistry CH3 Alanine (()Ala) Glutamic Acid (()Glu) Amino Acid Analysis - Agilent Restricted Page 3 June 24, 2010 Amino Acids – Zwitterionic Amino Acid Analysis - Agilent Restricted Page 4 June 24, 2010 Separation Considerations • Zwitterions - poor solubility near iso- …electric point • Most have poor UV absorbance • Derivatization – OPA, FMOC •Reduce polarity – increases retention in reversed-phase chromatoggpyraphy •Improve sensitivity – UV, Fluorescence • Detector; DAD, FLD, MS, ELSD Amino Acid Analysis - Agilent Restricted Page 5 June 24, 2010 Ortho Phthalaldehyde (OPA) and Fluorenylmethoxy chloroformate (FMOC) Reactions with Amines OPA O SR’ R’SH H NR +RNH2 H Room Temperature O Fluorescence: Ex 340nm, Em 450nm Non-fluorescent DAD: 338 , 10nm; Ref . 390 , 20nm Does not absorb at 338nm FMOC RR’NH - HCl + or Room Temperature RNH2 NRR’ or NHR Fluorescence: Ex 260nm, Em 325nm Fluorescent DAD: 262, 16nm; Ref. 324,8nm Absorbs at 262nm and Fluorescences at 324nm Group/Presentation Title Agilent Restricted Month ##, 200X Names and Order of Elution for OPA and FMOC Derivatives of Amino Acids Peak # AA Name AA Abbreviation Derivative Type Peak # AA Name AA Abbreviation Derivative Type Group/Presentation Title Agilent Restricted Month ##, 200X Agilent AAA Methods - They’ve Evolved • Automated Amino Acid Analysis – AminoQuant I & II (1987) •1090 •1100, Pub. -

Amino Acid Degradation
BI/CH 422/622 OUTLINE: OUTLINE: Protein Degradation (Catabolism) Digestion Amino-Acid Degradation Inside of cells Protein turnover Dealing with the carbon Ubiquitin Fates of the 29 Activation-E1 Seven Families Conjugation-E2 nitrogen atoms in 20 1. ADENQ Ligation-E3 AA: Proteosome 2. RPH 9 ammonia oxidase Amino-Acid Degradation 18 transamination Ammonia 2 urea one-carbon metabolism free transamination-mechanism to know THF Urea Cycle – dealing with the nitrogen SAM 5 Steps Carbamoyl-phosphate synthetase 3. GSC Ornithine transcarbamylase PLP uses Arginino-succinate synthetase Arginino-succinase 4. MT – one carbon metabolism Arginase 5. FY – oxidase vs oxygenase Energetics Urea Bi-cycle 6. KW – Urea Cycle – dealing with the nitrogen 7. BCAA – VIL Feeding the Urea Cycle Glucose-Alanine Cycle Convergence with Fatty acid-odd chain Free Ammonia Overview Glutamine Glutamate dehydrogenase Overall energetics Amino Acid A. Concepts 1. ConvergentDegradation 2. ketogenic/glucogenic 3. Reactions seen before The SEVEN (7) Families B. Transaminase (A,D,E) / Deaminase (Q,N) Family C. Related to biosynthesis (R,P,H; C,G,S; M,T) 1.Glu Family a. Introduce oxidases/oxygenases b. Introduce one-carbon metabolism (1C) 2.Pyruvate Family a. PLP reactions 3. a-Ketobutyric Family (M,T) a. 1-C metabolism D. Dedicated 1. Aromatic Family (F,Y) a. oxidases/oxygenases 2. a-Ketoadipic Family (K,W) 3. Branched-chain Family (V,I,L) E. Convergence with Fatty Acids: propionyl-CoA 29 N 1 Amino Acid Degradation • Intermediates of the central metabolic pathway • Some amino acids result in more than one intermediate. • Ketogenic amino acids can be converted to ketone bodies. -

Where Metal Ions Bind in Proteins (Metafloprotein/Protein Structure/Hydrophobicity Contrast Function) MASON M
Proc. Nadl. Acad. Sci. USA Vol. 87, pp. 5648-5652, August 1990 Biophysics Where metal ions bind in proteins (metafloprotein/protein structure/hydrophobicity contrast function) MASON M. YAMASHITA*t, LAURA WESSON*, GEORGE EISENMANt, AND DAVID EISENBERG*§ *Molecular Biology Institute and Department of Chemistry and Biochemistry, and *Department of Physiology, University of California, Los Angeles, CA 90024 Contributed by David Eisenberg, May 14, 1990 ABSTRACT The environments of metal ions (Li', Na', K+, Ag+, Cs+, Mg2+, Ca2+, Mn2+9 Cu2+, Zn2+) in proteins and other metal-host molecules have been examined. Regard- .. 0.00- ______ less ofthe metal and its precise pattern ofligation to the protein, there is a common qualitative feature to the bind site: the metal is ligated by a shell of hydrophilic atomic groups (con- E -0.01 Zn2+ taining oxygen, nitrogen, or sulfur atoms) and this hydrophilic shell is embedded within a larger shell of hydrophobic atomic - -0.02 groups (containing carbon atoms). That is, metals bind at centers of high hydrophobicity contrast. This qualitative ob- servation can be described analytically by the hydrophobicity 0 2 4 6 8 10 contrast function, C, evaluated from the structure. This func- tion is large and positive for a sphere of hydrophilic atomic 0.01 groups (characterized by atomic salvation parameters, Aar, having values < 0) at the center of a larger sphere of hydro- phobic atomic groups (characterized by Aor > 0). In the 23 0.00. metal-binding molecules we have examined, the maximum values of the contrast function lie near to observed metal binding sites. This suggests that the hydrophobicity contrast function may be useful for locating, characterizing, and de- - -0.01 signing metal binding sites in proteins. -

Safety Assessment of Α-Amino Acids As Used in Cosmetics
Safety Assessment of α-Amino Acids as Used in Cosmetics Status: Final Report for Public Distribution Release Date: October 5, 2012 Panel Meeting Date: September 10-11, 2012 The 2012 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; Ronald A. Hill, Ph.D. James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is F. Alan Andersen, Ph.D. This safety assessment was prepared by Christina L. Burnett, Scientific Analyst/Writer, and Bart Heldreth, Ph.D., Chemist CIR. © Cosmetic Ingredient Review 1101 17th Street, NW, Suite 412 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] 1 ABSTRACT The Cosmetic Ingredient Review Expert Panel (the Panel) reviewed the safety of α-amino acids, which function primarily as hair and skin conditioning agents in cosmetic products. The safety of α-amino acids as direct food additives has been well established based on extensive research through acute and chronic dietary exposures. The Panel focused its review on dermal irritation and sensitization data relevant to the use of these ingredients in topical cosmetics. The Panel concluded that α-amino acids were safe as cosmetic ingredients in the practices of use and concentration of this safety assessment. INTRODUCTION Amino acids and their salts are widely used as cosmetic ingredients, and function primarily as hair conditioning agents and skin conditioning agents (humectant and miscellaneous). -

THE CONDUCTIVITIES of AQUEOUS SOLUTIONS of GLYCINE, D,/-VALINE, and I-ASPARAGINE*
THE CONDUCTIVITIES OF AQUEOUS SOLUTIONS OF GLYCINE, d,/-VALINE, AND I-ASPARAGINE* B~ JOHN W. MEI-IL AND CARL L. A. SCHMIDT (From the Division of Biochemistry, Unirersity of California Medical School, Berkeley) (Accepted for publication, May 29, 1934) 30 years ago, Sir James Walker (1, 2) published two papers entitled, "The theory of amphoteric electrolytes," in which he discussed the conductivities of amphoteric electrolytes and their deviations from the Ostwald dilution law in terms of the mass law. Deriving the expres- sions for the various ion concentrations present in an ampholyte solu- tion, he showed why the attempt to apply the dilution law to such solutions is invalid. He was able to show that the conductivities calculated from the relations he had obtained were in agreement with Winkelblech's (3) data for the aminobermoic acids. With the exception of a single value for asparagine, the figures given by Walker are not concerned with the aliphatic amino acids. At the time there were no trustworthy data for the latter compounds. In 1895, Emile Franke (4) reported values for a-alanine up to a con- centration of 0.0156 ~r, and in 1905, Siegfried (5) published more measurements on alanine, this time from 0.125 to 1.0 ~. Values at isolated concentrations are also given by Bayliss (6). More recently the conductivity of glycine has been measured by Miyamoto and Schmidt (7). In connection with the measurement of the conductivity of asparagine, Walker pointed out the fact that the only criterion for the purity of such a compound is the attainment of constant con- ductivity on repeated purification, and that such a result was only obtained after the asparagine hadbeen recrystallized twenty-four times. -

The Diverse Functions of Non-Essential Amino Acids in Cancer
cancers Review The Diverse Functions of Non-Essential Amino Acids in Cancer Bo-Hyun Choi and Jonathan L. Coloff * Department of Physiology and Biophysics, University of Illinois Cancer Center, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] * Correspondence: coloff@uic.edu Received: 16 April 2019; Accepted: 10 May 2019; Published: 15 May 2019 Abstract: Far beyond simply being 11 of the 20 amino acids needed for protein synthesis, non-essential amino acids play numerous important roles in tumor metabolism. These diverse functions include providing precursors for the biosynthesis of macromolecules, controlling redox status and antioxidant systems, and serving as substrates for post-translational and epigenetic modifications. This functional diversity has sparked great interest in targeting non-essential amino acid metabolism for cancer therapy and has motivated the development of several therapies that are either already used in the clinic or are currently in clinical trials. In this review, we will discuss the important roles that each of the 11 non-essential amino acids play in cancer, how their metabolic pathways are linked, and how researchers are working to overcome the unique challenges of targeting non-essential amino acid metabolism for cancer therapy. Keywords: aspartate; asparagine; arginine; cysteine; glutamate; glutamine; glycine; proline; serine; cancer 1. Introduction It is now well established that tumors display different metabolic phenotypes than normal tissues [1]. The first observed and most studied metabolic phenotype of tumors is that of increased glucose uptake and glycolysis [2,3], a metabolic phenotype that is exploited in the clinic to image human tumors and metastases via 18flurodeoxyglucose positron emission tomography (18FDG-PET) [4].