1 Chlorofluorocarbons (Cfcs) Described As "Miracle Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Guide
Indoor Air Quality Tools for Schools REFERENCE GUIDE Indoor Air Quality (IAQ) U.S. Environmental Protection Agency Indoor Environments Division, 6609J 1200 Pennsylvania Avenue, NW Washington, DC 20460 (202) 564-9370 www.epa.gov/iaq American Federation of Teachers 555 New Jersey Avenue, NW Washington, DC 20001 (202) 879-4400 www.aft.org Association of School Business Officials 11401 North Shore Drive Reston, VA 22090 (703) 478-0405 www.asbointl.org National Education Association 1201 16th Steet, NW Washington, DC 20036-3290 (202) 833-4000 www.nea.org National Parent Teachers Association 330 North Wabash Avenue, Suite 2100 Chicago, IL 60611-3690 (312) 670-6782 www.pta.org American Lung Association 1740 Broadway New York, NY 10019 (212) 315-8700 www.lungusa.org EPA 402/K-07/008 I January 2009 I www.epa.gov/iaq/schools Introduction � U nderstanding the importance of good basic measurement equipment, hiring indoor air quality (IAQ) in schools is the professional assistance, and codes and backbone of developing an effective IAQ regulations. There are numerous resources program. Poor IAQ can lead to a large available to schools through EPA and other variety of health problems and potentially organizations, many of which are listed in affect comfort, concentration, and staff/ Appendix L. Use the information in this student performance. In recognition of Guide to create the best possible learning tight school budgets, this guidance is environment for students and maintain a designed to present practical and often comfortable, healthy building for school low-cost actions you can take to identify occupants. and address existing or potential air quality Refer to A Framework for School problems. -

Chlorofluorocarbons Chem 300A Tyler Jensen, Mackenzie Latimer, Jessie Luther, Jake Mcghee, Adeeb Noorani, Tyla Penner, & Maddy Springle
Chlorofluorocarbons Chem 300a Tyler Jensen, Mackenzie Latimer, Jessie Luther, Jake McGhee, Adeeb Noorani, Tyla Penner, & Maddy Springle 1.1: A Brief History of Chlorofluorocarbons Chlorofluorocarbons (CFCs), also known as Freons, were first synthesized in 1928 by Thomas Midgley Jr, who was working for General Motors trying to find a safe refrigerant to use in commercial applications. (Rosenbaum, n.d.). They are an anthropogenic compound containing fluorine, carbon, and chlorine atoms, and are classified as halocarbons. CFCs are a family of chemicals based upon hydrocarbon skeletons, where most hydrogens have been replaced with chlorine and/or fluorine atoms. They are chemically stable freons that are non-flammable, tasteless and odourless. CFCs are very volatile, which makes for ideal refrigerant gases, having a boiling point close to zero degrees (Rosenbaum, n.d) They were originally created to replace the toxic gases used in the late 1800’s and early 1900’s. Examples of the toxic gases replaced by CFCs as refrigerants are ammonia (NH3), methyl chloride (CH3Cl), and sulfur dioxide (SO2) (Wilkins, 1999). When first created dichlorodifloromethane was found to be less toxic than carbon dioxide, and as non-flammable as carbon tetrachloride (Midgley & Henne, 1930). The non-toxic, non-flammable, and non-reactive properties of CFCs made them ideal for use as refrigerants. CFCs were used in many developed countries for consumption and production as they were inflammable and non-toxic towards humanity. Chlorofluorocarbons can be used as refrigerants, cleaning agents, foaming agents, and propellants for aerosol sprays (Welch, n.d.). In 1974, Two University of California chemists, Professor F. Sherwood Rowland and Dr. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

Use of Chlorofluorocarbons in Hydrology : a Guidebook
USE OF CHLOROFLUOROCARBONS IN HYDROLOGY A Guidebook USE OF CHLOROFLUOROCARBONS IN HYDROLOGY A GUIDEBOOK 2005 Edition The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GREECE PANAMA ALBANIA GUATEMALA PARAGUAY ALGERIA HAITI PERU ANGOLA HOLY SEE PHILIPPINES ARGENTINA HONDURAS POLAND ARMENIA HUNGARY PORTUGAL AUSTRALIA ICELAND QATAR AUSTRIA INDIA REPUBLIC OF MOLDOVA AZERBAIJAN INDONESIA ROMANIA BANGLADESH IRAN, ISLAMIC REPUBLIC OF RUSSIAN FEDERATION BELARUS IRAQ SAUDI ARABIA BELGIUM IRELAND SENEGAL BENIN ISRAEL SERBIA AND MONTENEGRO BOLIVIA ITALY SEYCHELLES BOSNIA AND HERZEGOVINA JAMAICA SIERRA LEONE BOTSWANA JAPAN BRAZIL JORDAN SINGAPORE BULGARIA KAZAKHSTAN SLOVAKIA BURKINA FASO KENYA SLOVENIA CAMEROON KOREA, REPUBLIC OF SOUTH AFRICA CANADA KUWAIT SPAIN CENTRAL AFRICAN KYRGYZSTAN SRI LANKA REPUBLIC LATVIA SUDAN CHAD LEBANON SWEDEN CHILE LIBERIA SWITZERLAND CHINA LIBYAN ARAB JAMAHIRIYA SYRIAN ARAB REPUBLIC COLOMBIA LIECHTENSTEIN TAJIKISTAN COSTA RICA LITHUANIA THAILAND CÔTE D’IVOIRE LUXEMBOURG THE FORMER YUGOSLAV CROATIA MADAGASCAR REPUBLIC OF MACEDONIA CUBA MALAYSIA TUNISIA CYPRUS MALI TURKEY CZECH REPUBLIC MALTA UGANDA DEMOCRATIC REPUBLIC MARSHALL ISLANDS UKRAINE OF THE CONGO MAURITANIA UNITED ARAB EMIRATES DENMARK MAURITIUS UNITED KINGDOM OF DOMINICAN REPUBLIC MEXICO GREAT BRITAIN AND ECUADOR MONACO NORTHERN IRELAND EGYPT MONGOLIA UNITED REPUBLIC EL SALVADOR MOROCCO ERITREA MYANMAR OF TANZANIA ESTONIA NAMIBIA UNITED STATES OF AMERICA ETHIOPIA NETHERLANDS URUGUAY FINLAND NEW ZEALAND UZBEKISTAN FRANCE NICARAGUA VENEZUELA GABON NIGER VIETNAM GEORGIA NIGERIA YEMEN GERMANY NORWAY ZAMBIA GHANA PAKISTAN ZIMBABWE The Agency’s Statute was approved on 23 October 1956 by the Conference on the Statute of the IAEA held at United Nations Headquarters, New York; it entered into force on 29 July 1957. The Headquarters of the Agency are situated in Vienna. -
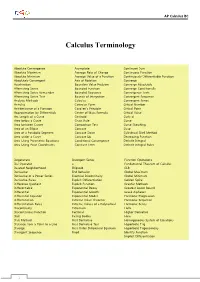
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Applied and Fundamental Heterogeneous Catalysis Studies
APPLIED AND FUNDAMENTAL HETEROGENEOUS CATALYSIS STUDIES ON HYDRODECHLORINATION OF TRICHLOROETHYLENE AND STEAM REFORMING OF ETHANOL DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Hyuntae Sohn, M.S. Graduate Program in Chemical Engineering The Ohio State University 2016 - Dissertation Committee - Prof. Umit S. Ozkan, Advisor Prof. Andre Palmer Prof. Lisa Hall Prof. T.V. (Babu) RajanBabu Copyright by Hyuntae Sohn 2016 ABSTRACT The dissertation herein reports heterogeneous catalysis studies conducted on two different projects, (1) Hydrodechlorination of trichloroethylene and (2) Ethanol steam reforming. The former is associated with the process for trichloroethylene waste treatment and groundwater remediation technology whereas the latter pertains to hydrogen production. The presented work involves not only investigations on the catalytic activity, but also fundamental studies to understand how and why a catalyst works for a particular reaction. This dissertation is composed of two major parts. (1) Part I (Chapter 1-4): Trichloroethylene (TCE) is a chlorinated hydrocarbon solvent which has been widely used as a vapor degreaser for metal cleaning. TCE contains high level of toxicity, also known as a carcinogen. Recently, contamination of groundwater due to untreated TCE is a rapidly rising environmental issue, affecting the drinking water quality. The current waste treatment process for removal of TCE are mostly based on adsorption and extraction techniques. However, these processes do not provide a complete destruction of the TCE chemical structure; hence, it requires an additional incinerator to convert TCE into non harmful products. Hydrodechlorination (HDC) of TCE is a catalytic chemical reaction where TCE is converted to ethane and hydrochloric acid using hydrogen as a reducing agent. -

THOMAS MIDGLEY, JR., and the INVENTION of CHLOROFLUOROCARBON REFRIGERANTS: IT AIN’T NECESSARILY SO Carmen J
66 Bull. Hist. Chem., VOLUME 31, Number 2 (2006) THOMAS MIDGLEY, JR., AND THE INVENTION OF CHLOROFLUOROCARBON REFRIGERANTS: IT AIN’T NECESSARILY SO Carmen J. Giunta, Le Moyne College The 75th anniversary of the first public Critical readers are well aware description of chlorofluorocarbon (CFC) of the importance of evaluating refrigerants was observed in 2005. A sources of information. For ex- symposium at a national meeting of ample, an article in Nature at the the American Chemical Society (ACS) end of 2005 tested the accuracy on CFCs from invention to phase-out of two encyclopedias’ entries on a (1) and an article on their invention sample of topics about science and and inventor in the Chemical Educa- history of science (3). A thought- tor (2) marked the occasion. The story ful commentary published soon of CFCs—their obscure early days as afterwards raised questions on just laboratory curiosities, their commercial what should count as an error in debut as refrigerants, their expansion into assessing such articles: omissions? other applications, and the much later disagreements among generally re- discovery of their deleterious effects on liable sources (4)? Readers of his- stratospheric ozone—is a fascinating torical narratives who are neither one of science and society well worth practicing historians nor scholarly telling. That is not the purpose of this amateurs (the category to which I article, though. aspire) may find an account of a Thomas Midgley, Jr. historical research process attrac- This paper is about contradictory Courtesy Richard P. Scharchburg tive. As a starting point, consider sources, foggy memories, the propaga- Archives, Kettering University the following thumbnail summary tion of error, and other obstacles to writ- of the invention. -

Overview of Fluorocarbons
Three Bond Technical News Issued Dec. 20, 1989 An Overview of Fluorocarbons Introduction Currently, chlorofluorocarbons are garnering a lot of Three Bond Co,. Ltd. has been working night and day in attention around the world. Chlorofluorocarbons are used in search of alternatives to chlorofluorocarbons. In the future, many of our products and when we look at the future of products that do not contain any chlorofluorocarbons will chlorofluorocarbons in the world, and in Japan, we can replace products that now contain chlorofluorocarbons. To foresee that their use will be strictly limited or eliminated all ensure these products reach our customers as smoothly as together. possible, it is important that they have a good understanding of the issues surrounding chlorofluorocarbons. Whatever the case, Three Bond Co., Ltd. is obligated to provide our customers with products that have the same functionality as chlorofluorocarbons. Contents Introduction ................................................................................................................................................................1 1. What is a fluorocarbon? .......................................................................................................................................2 2. Properties of fluorocarbons ..................................................................................................................................2 3. Types of fluorocarbons.........................................................................................................................................2 -
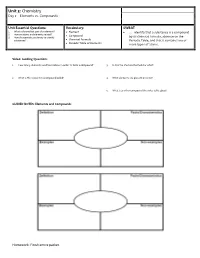
Unit 3: Chemistry Day 2 – Elements Vs
Unit 3: Chemistry Day 2 – Elements vs. Compounds Unit Essential Questions: Vocabulary: SWBAT 1. What is the smallest part of an element? Element … identify that a substance is a compound 2. How are atoms and elements related? Compound 3. How do scientists use density to identify by its chemical formula, absence on the substances? Chemical Formula Periodic Table, and that it contains two or Periodic Table of Elements more types of atoms. Video: Guiding Questions 1. How many elements need to combine in order to form a compound? 3. H2O is the chemical formula for what? 2. What is the recipe for a compound called? 4. What elements are present in water? 5. What is another compound the video talks about? GUIDED NOTES: Elements and Compounds Homework: Finish entire packet. STOP AND JOT: What is the difference between an element and a compound? Chemical Formulas Compounds are represented by _________________________________ _____________________________________. Each capital letter represents _______________ element. ____________________________________ represent the number of atoms of each element. Examples: H2O Si3N4 Number of elements: _____________________ Number of elements: _____________________ Number of atoms for each element: Number of atoms for each element: ______________________ ______________________ ______________________ ________________________ How many elements are in How many atoms of each How many elements are in How many atoms of each the compound H3PO4? element is present in the the compound C2H5OH? element is present in the compound, HNO3? compound, C2H4O2? Three ways to identify a Compound: 1. Made of more than one _________________________. 2. Not located on the _____________________________ __________________________ _________________ ________________________________. 3. Represented by a ____________________________________ ____________________________________. -

E.W. Dijkstra Archive: on the Cruelty of Really Teaching Computing Science
On the cruelty of really teaching computing science Edsger W. Dijkstra. (EWD1036) http://www.cs.utexas.edu/users/EWD/ewd10xx/EWD1036.PDF The second part of this talk pursues some of the scientific and educational consequences of the assumption that computers represent a radical novelty. In order to give this assumption clear contents, we have to be much more precise as to what we mean in this context by the adjective "radical". We shall do so in the first part of this talk, in which we shall furthermore supply evidence in support of our assumption. The usual way in which we plan today for tomorrow is in yesterday’s vocabulary. We do so, because we try to get away with the concepts we are familiar with and that have acquired their meanings in our past experience. Of course, the words and the concepts don’t quite fit because our future differs from our past, but then we stretch them a little bit. Linguists are quite familiar with the phenomenon that the meanings of words evolve over time, but also know that this is a slow and gradual process. It is the most common way of trying to cope with novelty: by means of metaphors and analogies we try to link the new to the old, the novel to the familiar. Under sufficiently slow and gradual change, it works reasonably well; in the case of a sharp discontinuity, however, the method breaks down: though we may glorify it with the name "common sense", our past experience is no longer relevant, the analogies become too shallow, and the metaphors become more misleading than illuminating. -
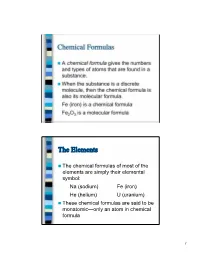
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom.