GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
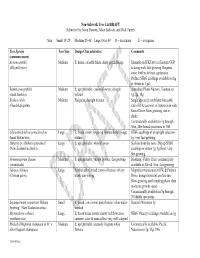
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Cyanogenic Glycosides • Isothiocyanate Glycosides • Phenolic & Aldehyde Glycosides • Coumarins - Bitter Principles • Terpenes and Terpenoids • Tannins Glycosides
Dr. Pran Kishore Deb and Dr. Yousef Abusamra Phytotherapy • Phytotherapy is the study of the use of extracts of natural origin as medicines or health-promoting agents. • Traditional phytotherapy is a synonym for herbalism and regarded as alternative medicine by much of Western medicine. PART – 1 • Glycosides • Saponin glycosides • Flavonoid glycosides • Anthocyanidin • Cyanogenic glycosides • Isothiocyanate glycosides • Phenolic & Aldehyde glycosides • Coumarins - Bitter principles • Terpenes and Terpenoids • Tannins Glycosides • They are compounds containing a carbohydrate (sugar) and a noncarbohydrate residue in the same molecule. • The carbohydrate residue is attached by an acetal linkage at carbon atom 1 to a noncarbohydrate residue. • The carbohydrate (sugar) component is called the GLYCONE. • The noncarbohydrate component is known as the AGLYCONE. • The sugar moiety can be joined to the aglycone in various ways: 1. Oxygen (O-glycoside) 2. Sulphur (S-glycoside) 3. Nitrogen (N-glycoside) 4. Carbon (C-glycoside) Classification of Glycosides • The aglycone may be methyl alcohol, glycerol, a sterol, a phenol, etc. • Based on the chemical nature of the aglycone group, glycosides can be classified as follows: 5 SAPONIN GLYCOSIDES SAPONIN GLYCOSIDES • Saponins on hydrolysis yield an aglycone known as "sapogenin". • Saponin glycosides are divided into 2 types based on the chemical structure of their aglycones (sapogenins). Classification of Saponins: According to the nature of the aglycone: 1. Neutral saponins or Steroidal saponins with spiroketal side chains. 2. Acid saponins or Triterpenoidal saponins Sug-O Sug-O Steroidal Saponins Triterpenoidal Saponins 7 Steroidal Saponins • Neutral steroidal glycosides which contain spiroketal side chain. • Two rings E and F called ketal because they are attached through two oxygen atoms and called spiral because they are not on the same level. -

Quillaja Saponaria (Molina) Extracts Inhibits in Vitro Piscirickettsia Salmonis Infections
animals Article Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections Hernán Cañon-Jones 1,* , Hernán Cortes 2, Mario Castillo-Ruiz 3,4 , Trinidad Schlotterbeck 5 and Ricardo San Martín 5 1 Núcleo de Investigación Aplicada en Ciencias Veterinarias y Agronómicas, Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago 7500975, Chile 2 Desert King Chile, Viña del Mar 2420505, Chile; [email protected] 3 Escuela de Química y Farmacia, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile; [email protected] 4 Departamento de Ciencias Químicas y Biológicas, Facultad de Ciencias de la Salud, Universidad Bernardo O Higgins, Santiago 8370993, Chile 5 Saponin Research Center, Santiago 7510132, Chile; [email protected] (T.S.); [email protected] (R.S.M.) * Correspondence: [email protected] Received: 15 September 2020; Accepted: 10 November 2020; Published: 3 December 2020 Simple Summary: Bacterial diseases causes massive mortalities in aquaculture and antibiotic use remains the main measure to keep these under control. Pisciricketssia salmonis, an intracellular bacterium only present in Chile, produces high mortalities in farmed salmon and is currently the main reason for using antimicrobials compared to other salmon-producing countries such as Norway. Environmental and antimicrobial resistance concerns have been raised by the local and global public and society, although no scientific evidence has demonstrated such an impact. Thus, there is a constant search for new alternatives that can complement or reduce the use of antimicrobial in intensive salmon farming. Phytochemicals such as saponins from Quillaja saponaria extracts have been proven to prevent and control diseases in other animal production systems. -

Nemomex Nematicide SAFETY DATA SHEET
NemOmex Nematicide SAFETY DATA SHEET Section 1: Identification of the substance/mixture and of the company/undertaking 1.1.1.2. 1.1. Product name : NemOmex Nematicide Identification : EPA Reg. No. 82572-1-86868 CAS number : 68990-67-0 EINECS number : 273-620-4 USA (FDA) : E999 21 CFR 172.510. FEMA GRAS NUMBER 2973. 1.3.1.4. 1.2. Relevant identified uses of substance or mixture and uses advised against. Wetting agent. 1.5.1.6. 1.3. Details of supplier for the safety data sheet. Omex Agrifluids, Inc. 1675 Dockery Avenue Selma, CA 93662 1.7.1.8. 1.4. Emergency telephone number. 1-800-424-9300 Section 2: Hazards identification 2.1. Product definition: Quillaja saponaria wood extract liquid. 2.2. Classification of the substance or mixture. According to Directive criteria, 1999/45/EC and 67/548/EEC the following amendments thereof: Xi : Irritant. R Phases : R36 Irritating to eyes. S Phrases : S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Regulation criteria 1272/2008 (CLP/GHS): : warning. Hazard Statements : H320. Causes eye irritation. Precautionary Statements: P264 : Wash with water the parts of your body that had contact with the product. P280 : Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 : IF IN EYES. Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do so, continue rinsing. P314 : Get medical advice/attention if you feel unwell. P362 : Take off contaminated clothing and wash before reuse. Page 1 of 9 NemOmex Nematicide P501 : Dispose of contents/container in accordance with applicable regulations. -

Contributions to the Solution of Phylogenetic Problem in Fabales
Research Article Bartın University International Journal of Natural and Applied Sciences Araştırma Makalesi JONAS, 2(2): 195-206 e-ISSN: 2667-5048 31 Aralık/December, 2019 CONTRIBUTIONS TO THE SOLUTION OF PHYLOGENETIC PROBLEM IN FABALES Deniz Aygören Uluer1*, Rahma Alshamrani 2 1 Ahi Evran University, Cicekdagi Vocational College, Department of Plant and Animal Production, 40700 Cicekdagi, KIRŞEHIR 2 King Abdulaziz University, Department of Biological Sciences, 21589, JEDDAH Abstract Fabales is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae), Polygalaceae, Surianaceae and Quillajaceae. The monophyly of the order is supported strongly by several studies, although interfamilial relationships are still poorly resolved and vary between studies; a situation common in higher level phylogenetic studies of ancient, rapid radiations. In this study, we carried out simulation analyses with previously published matK and rbcL regions. The results of our simulation analyses have shown that Fabales phylogeny can be solved and the 5,000 bp fast-evolving data type may be sufficient to resolve the Fabales phylogeny question. In our simulation analyses, while support increased as the sequence length did (up until a certain point), resolution showed mixed results. Interestingly, the accuracy of the phylogenetic trees did not improve with the increase in sequence length. Therefore, this study sounds a note of caution, with respect to interpreting the results of the “more data” approach, because the results have shown that large datasets can easily support an arbitrary root of Fabales. Keywords: Data type, Fabales, phylogeny, sequence length, simulation. 1. Introduction Fabales Bromhead is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae) Juss., Polygalaceae Hoffmanns. -

Recruitment Dynamics of the Relict Palm, Jubaea Chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile
RESEARCH ARTICLE Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile Marina Fleury1,2*, Wara Marcelo2¤, Rodrigo A. Vásquez2, Luis Alberto González1, Ramiro O. Bustamante2 1 Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, Santiago, Chile, 2 Instituto de Ecología y Biodiversidad, Departamento de Ciencias Ecológicas, Universidad de Chile, Santiago, Chile ¤ Current address: Instituto de Conservación, Biodiversidad y Territorio, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Valdivia, Chile * [email protected] OPEN ACCESS Citation: Fleury M, Marcelo W, Vásquez RA, Abstract González LA, Bustamante RO (2015) Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Shrubs can have a net positive effect on the recruitment of other species, especially relict Intricate and Pervasive Effects of Invasive Herbivores species in dry-stressful conditions. We tested the effects of nurse shrubs and herbivory and Nurse Shrubs in Central Chile. PLoS ONE 10(7): defoliation on performance (survival and growth) of nursery-grown seedlings of the largest e0133559. doi:10.1371/journal.pone.0133559 living palm, the relict wine palm Jubaea chilensis. During an 18-month period, a total of Editor: Jin-Song Zhang, Institute of Genetics and more than 300 seedlings were exposed to of four possible scenarios produced by indepen- Developmental Biology, Chinese Academy of Sciences, CHINA dently weakening the effects of nurse shrubs and browsers. The experiment followed a two- way fully factorial design. We found consistent differences in survival between protected Received: March 2, 2015 and unprotected seedlings (27.5% and 0.7%, respectively), and herbivory had a dramatic Accepted: June 28, 2015 and overwhelmingly negative effect on seedling survival. -

FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast
FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast Beverages Entrée - Bowls/Mac/Flatbread Pizza Kids Dressings Pastries & Sweets Salads Sandwiches Sides Smoothies Souffles Soups Spreads Grab N Go Catering ©2021 Panera Bread. All Rights Reserved. Bakery - Cafe Nutrition, Ingredient, and Allergen Information Effective 1/13/2021 Version 2 Bagels Asiago Cheese Bagel g) m ( l mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat (g) m i i i ASIAGO CHEESE BAGEL (ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, s ra u i ar REDUCED IRON, THIAMINE MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, ASIAGO CHEESE u (g) ole lor lor rbs (g) g d t a a h a (PASTEURIZED MILK, CHEESE CULTURE, SALT, MICROBIAL AND ANIMAL ENZYMES, POWDERED nts Servin C C Fa Sat Tran C So C Fiber (g) Su Prote CELLULOSE [TO PREVENT CAKING]), BROWN SUGAR, SALT, DOUGH IMPROVER (MALTED e i WHEAT FLOUR, ENRICHED WHEAT FLOUR [NIACIN, REDUCED IRON, THIAMINE MONONITRATE, d RIBOFLAVIN, FOLIC ACID], INACTIVATED YEAST, ACEROLA EXTRACT, FUNGAL ENZYMES), YEAST e (YEAST, SORBITAN MONOSTEARATE, ASCORBIC ACID)), ASIAGO CHEESE (PASTEURIZED MILK, 1 Bagel 320 50 5 3 0 15 530 55 2 4 14 CHEESE CULTURES, SALT, MICROBIAL & ANIMAL ENZYMES, POWDERED CELLULOSE [TO PREVENT CAKING]). Ingr CONTAINS MILK, WHEAT. Allergens * Blueberry Bagel g) m ) ( l g mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat ( m i i i ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, REDUCED IRON, THIAMINE n s ra u i i ar MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, BLUEBERRY FLAVORED -

Quillaia-Saponins-Summary-Report-Committee-Veterinary-Medicinal-Products En.Pdf
The European Agency for the Evaluation of Medicinal Products EMEA/MRL/055/95-FINAL February 1996 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS QUILLAIA SAPONINS SUMMARY REPORT 1. Saponins are some of the most common constituents of plants and are found in over 500 plant genera. Saponins occur in some plants used as human foods, e.g. potatoes, tomatoes, onions, soya, beans, peas, tea, oats and some herbs and spices. They also occur in some animal forage crops, e.g. alfalfa. Chemically, saponins are glycosides with one or more mono- or oligo- saccharide groups in glycosidic links with a non-polar aglycone, usually of a steroid or triterpenoid structure. 2. Saponins are surface active agents which produce a soapy lather when they are shaken with water. Most commercially- produced "saponin" or "quillaia extract" is an aqueous extract of the dried inner bark of the tree Quillaia saponaria Molina. The bark extract is widely used in toiletry preparations and in the food and beverage industries, as an emulsifier and foaming agent. In soft drinks, quillaia extracts are used at concentrations of up to 200 mg/kg. Quillaia extracts are frequently included in cough mixtures for human use. 3. Pharmacopoeial monographs for Quillaia have been included in the British and French Pharmacopoeias. 4. Quillaia saponins have immunostimulant properties. In veterinary medicines, the main use is as a vaccine adjuvant. The adjuvant component is a purified form of quillaia extract, of uniform composition, usually denoted "Quil A". A typical parenteral dose of vaccine contains 350 mg of saponins. 5. Saponins possess a number of pharmacological properties of which the most important is their ability to lyse erythrocytes in vivo. -

Evaluation of Certain Food Additives: Sixty-Fifth Reports of the Joint FAO/WHO Expert Committee On
WHO Technical Report Series 934 EVALUATION OF CERTAIN FOOD ADDITIVES This report represents the conclusions of a Joint FAO/WHO Expert EVALUATION OF CERTAIN Committee convened to evaluate the safety of various food additives, with a view to recommending acceptable daily intakes (ADIs) and FOOD ADDITIVES to prepare specifications for the identity and purity of food additives. The first part of the report contains a general discussion of the principles governing the toxicological evaluation of food additives (including flavouring agents), assessments of intake, and the establishment and revision of specifications for food additives. A summary follows of the Committee’s evaluations of toxicological and intake data on various specific food additives (Beeswax, Candelilla wax, Calcium L-5-Methyltetrahydrofolic acid (L-5-MTHF), Phospholipase A1 from Fusarium venenatum expressed in Aspergillus oryzae, Pullulan, Quillaia extract Type 1, Quillaia extract Type 2) and seven groups Sixty-fifth report of the joint of flavouring agents. Annexed to the report are tables summarizing FAO/WHO Expert Committee on food additives the Committee’s recommendations for ADIs of the food additives, recommendations on the flavouring agents considered, changes in the status of specifications, and further information requested or desired. WHO Technical Report Series – 934 ISBN 92 4 120934 8 The World Health Organization was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective and reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. -

Seasonal Analysis of Saponin Content of Leaves of Young Quillaja Saponaria Trees from a Plantation
PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE ESCUELA DE INGENIERIA SEASONAL ANALYSIS OF SAPONIN CONTENT OF LEAVES OF YOUNG QUILLAJA SAPONARIA TREES FROM A PLANTATION TRINIDAD SCHLOTTERBECK SUAREZ Thesis submitted to the Office of Research and Graduate Studies in partial fulfillment of the requirements for the Degree of Master of Science in Engineering Advisor: RICARDO SAN MARTIN Santiago de Chile, march, 2015 © 2015, Trinidad Schlotterbeck PONTIFICIA UNIVERSIDAD CATOLICA DE CHILE ESCUELA DE INGENIERIA SEASONAL ANALYSIS OF SAPONIN CONTENT OF LEAVES YOUNG QUILLAJA SAPONARIA TREES IN A PLANTATION TRINIDAD SCHLOTTERBECK SUÁREZ Members of the Committee: RICARDO SAN MARTIN LORETO VALENZUELA MARIO CASTILLO JUAN DE DIOS ORTUZAR Thesis submitted to the Office of Research and Graduate Studies in partial fulfillment of the requirements for the Degree of Master of Science in Engineering Santiago de Chile, march, 2015 To my family, for their unconditional support and company. ii ACKNOWLEDGEMENTS First, I want to thank all my family for their support during all the years at university. Their patience and comprehension helped me finish my studies. I also would like to thank my great advisor, Ricardo San Martín, for his guidance and permanent help during all the activities done for my master’s research. I thank the constant assistance of Mario Castillo, especially during sample collections and analyses developments. I appreciate the commentaries and revisions of Hernán Cañón. I also thank Loreto Valenzuela for teaching me how to structure my research. I specially thank Mr. Hugo Vera and Mr. Patricio Guzman, for allowing me to do the sample collections of their Quillaja Farm and all the analysis required for the development of my master’s research. -

Biological Activities and Distribution of Plant Saponins S.G
Journal of Ethnopharmacology 94 (2004) 219–243 Review Biological activities and distribution of plant saponins S.G. Sparg, M.E. Light, J. van Staden∗ Research Centre for Plant Growth and Development, University of KwaZulu-Natal, Pietermaritzburg, Private Bag X01, Scottsville 3209, South Africa Received 9 February 2004; received in revised form 28 May 2004; accepted 29 May 2004 Abstract Plant saponins are widely distributed amongst plants and have a wide range of biological properties. The more recent investigations and findings into their biological activities were summarized. Isolation studies of saponins were examined to determine which are the more commonly studied plant families and in which families saponins have been identified. © 2004 Elsevier Ireland Ltd. All rights reserved. Keywords: Plant saponins; Triterpenoid; Steroidal; Biological activity; Distribution 1. Introduction most important traditional oriental medicines and is now used worldwide (Fukuda et al., 2000). Saponins are said to Saponins are a vast group of glycosides, widely distributed make up the active major constituents of ginseng. The genus in higher plants. Their surface-active properties are what Bupleurum is officially listed in Chinese and Japanese Phar- distinguish these compounds from other glycosides. They macopoeias are used in Asian traditional medicines to treat dissolve in water to form colloidal solutions that foam upon different ailments. The dry roots of Bupleurum fruticescens shaking (Tyler et al., 1981). Saponin containing plants are L. (Apiaceae) are traditionally used to treat disorders asso- sought after for use in household detergents (sapo, onis ciated with inflammation. The main anti-inflammatory com- = soap) (Bruneton, 1995). One such example is the soapwort pounds found in Bupleurum fruticescens are saikosaponins (Saponaria officinalis L.), which has been widely used for (Just et al., 1998). -

Jubaea 2018 Autumn
JubaeaFriends of Geelong Botanic Gardens Inc Newsletter Volume 18 Issue 2 Mar/ Apr/ May 2018 OUR PERENNIAL BORDER This border is one of the most photographed areas of our sourced from Wirruna Nursery, other selected nurseries, private Geelong Botanic Gardens and marks the division between the gardens and the GBG. original Botanic Gardens or nursery area and the 1959 Gardens Our gardeners are looking for impact, both in colour and form. extension. The inspiration came after a talk to the Friends in Plants are grouped for different effect and include a diversity 1994 about a similar border at the Royal Botanic Gardens in and colour of foliage as well as the flowers. Melbourne, by the then curator, Donna Sommerville. The border is intended to showcase plants that do well in the Our Friends, under the guidance of nurserywoman Judy Bailey Geelong region with a minimum of water, but the recent and the support of GBG Director Ian Rogers and his staff, addition of irrigation to the gardens has made a difference. It is completely reworked the beds on either side of the central an absolute picture for many months over Spring, Summer and pathway. This partnership between the Friends and the GBG Autumn. gardens staff continues to this day, nearly thirty years later. The border team of Friends’ Gardeners meet every Wednesday A further refurbishment was undertaken in 2000 when the morning and work solidly planting, weeding, cutting back and south border was increased in size by a third. Plants were dividing plants. Any excess is potted up by the Growing Friends for sale in the For the Friends’ 25th Anniversary the group produced the now nursery.