Plant Extracts Containing Saponins Affects the Stability and Biological
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
GRAS Notice (GRN) No. 903 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory NATUREX• part of Givaudan 'fR1@CG~nill~(jJ) 07 January 2020 JAN 1 5 2020 Dr. Paulette Gaynor FOOD OF, ,Li,; UF L ADDITIVE SAFETY Office of Food Additive Safety (HFS-200) __J Center for Food Safety and Applied Nutrition (CFSAN) Food and Drug Administration 5001 Campus Drive College Park, MD 20740 USA Dear Dr. Gaynor: Re: GRAS Notice for Qulllaia Extract Type 2 In accordance with 21 CFR §170 Subpart E consisting of§§ 170.203 through 170.285, Naturex SA (250 rue Pierre Bayle, BP 81218 - 84911, Avignon, Cedex 9, France], as the notifier, is submitting one hard copy and one electronic copy (on CD) of the GRAS Notice for quillaia extract type 2, containing all data and lnfonnation supporting the company's conclusion that quillaia extract type 2 is GRAS on the basis of scientific procedures, for use in specified conventional food and beverage products across multiple categories; these food uses of quillaia extract type 2 are therefore not subject to the premarket approval requirements of the Federal Food, Drug and Cosmetic Act. Information setting forth the basis for Naturex's GRAS conclusion, as well as a consensus opinion of an independent panel of experts, also are enclosed for review by the agency. I certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using Symantec Endpoint Protection 12.1.4. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. -
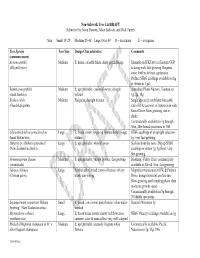
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Clean Lean Protein Technical Bulletin
SEPT 2015 CLEAN LEAN PROTEIN TECHNICAL BULLETIN ULTRA-HIGH BIOLOGICAL VALUE GOLDEN PEA PROTEIN, ALKALINE, NO ADDED MALTODEXTRIN, LACTOSE, POLYOLS OR FODMAPS All 9 essential amino acids. High glutamine. Very low carbs and low fat. No fillers, no artificial additives, preservatives, sweeteners, flavours or colours DISCLAIMER For educational and informational use by practitioners and businesses only The information in this Technical Bulletin is intended exclusively for business- to-business use, and not for the end consumer. The Technical Bulletin has been compiled independently by ANH Consultancy Ltd (The Atrium, Curtis Road, Dorking, Surrey RH4 1XA, UK) to provide education and information specifically for qualified healthcare practitioners, retailers and sport and fitness professionals with interests in the NuZest product range or ingredients contained within it. Accordingly, NuZest can accept no responsibility for the quality or accuracy of the material contained herein. The information and any implied health claims made are not necessarily authorised by national or EU authorities for communication to the end consumer. GLUTEN FREE • DAIRY FREE • LECTIN FREE • SOY FREE • GMO FREE T 1300 575 121 W nuzest.com.au E [email protected] facebook.com/nuzest.aus NUZEST CLEAN LEAN PROTEIN CONTENTS 1 INTRODUCTION & PRODUCT JUSTIFICATION 2 PEA PROTEIN ISOLATE 3 NUTRITIONAL PROFILE 4 MANAGING THE BODY’S AMINO ACID POOL 6 SUPPORTING ATHLETES & ACTIVE LIFESTYLES 8 WHAT’S IN CLEAN LEAN PROTEIN? 10 CLEAN LEAN PROTEIN 11 REFERENCES 12 T 1300 575 121 W nuzest.com.au E [email protected] facebook.com/nuzest.aus INTRODUCTION & PRODUCT JUSTIFICATION 2 NUZEST’S CLEAN LEAN PROTEIN (CLP) IS A UNIQUE, VEGETARIAN AND VEGAN, PROTEIN CHARACTERISED BY ITS TASTE (4 NATURAL FLAVOURS AND AN UNFLAVOURED), LACK OF FILLERS OR FOOD ADDITIVES, AND VERY HIGH DIGESTIBILITY. -

Quillaja Saponaria (Molina) Extracts Inhibits in Vitro Piscirickettsia Salmonis Infections
animals Article Quillaja saponaria (Molina) Extracts Inhibits In Vitro Piscirickettsia salmonis Infections Hernán Cañon-Jones 1,* , Hernán Cortes 2, Mario Castillo-Ruiz 3,4 , Trinidad Schlotterbeck 5 and Ricardo San Martín 5 1 Núcleo de Investigación Aplicada en Ciencias Veterinarias y Agronómicas, Facultad de Medicina Veterinaria y Agronomía, Universidad de Las Américas, Santiago 7500975, Chile 2 Desert King Chile, Viña del Mar 2420505, Chile; [email protected] 3 Escuela de Química y Farmacia, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile; [email protected] 4 Departamento de Ciencias Químicas y Biológicas, Facultad de Ciencias de la Salud, Universidad Bernardo O Higgins, Santiago 8370993, Chile 5 Saponin Research Center, Santiago 7510132, Chile; [email protected] (T.S.); [email protected] (R.S.M.) * Correspondence: [email protected] Received: 15 September 2020; Accepted: 10 November 2020; Published: 3 December 2020 Simple Summary: Bacterial diseases causes massive mortalities in aquaculture and antibiotic use remains the main measure to keep these under control. Pisciricketssia salmonis, an intracellular bacterium only present in Chile, produces high mortalities in farmed salmon and is currently the main reason for using antimicrobials compared to other salmon-producing countries such as Norway. Environmental and antimicrobial resistance concerns have been raised by the local and global public and society, although no scientific evidence has demonstrated such an impact. Thus, there is a constant search for new alternatives that can complement or reduce the use of antimicrobial in intensive salmon farming. Phytochemicals such as saponins from Quillaja saponaria extracts have been proven to prevent and control diseases in other animal production systems. -

Nutrition & Supplements Catalog
2 019 — 2020 But wait, there’s MORE! We can’t publish all our items in one catalog—it takes SIX catalogs! Find details about the different AZURE STANDARD catalogs we offer throughout AzureStandard.com the year on page 2. Nutrition & Supplements Catalog 971-200-8350 | 971-200-8350 Nutrition & Supplements THE ITEMS IN THIS CATALOG meet a high threshold for quality. Azure requires transparency from all the companies we work with, and we only choose suppliers that produce real foods made with only natural ingredients. That means you get nutrient-rich foods that are minimally processed with no artificial additives, no preservatives, non-GMO, no MSG, no artificial colors or flavors. That doesn’t mean every item is organic. Some are made with organic ingredients but are not Certified Organic. Some go far beyond organic. They all are earth-friendly, non-GMO and meticulously chosen for their healthful qualities. Azure’s Unacceptable Ingredient List for Food Items: Artificial Colors Certified Colors Pork Products Artificial Flavors Fluoride Shellfish Products (except Artificial Nitrates/Nitrites in Genetically Modified when used as an ingredient Meat Products Organisms (GMOs) in nutritional supplements) Artificial Preservatives Monosodium Glutamate Alcohol Artificial Sweeteners (MSG) Tobacco Bleached Flour Refined Conventional Sugars A Word about “Natural” Flavorings: Azure is currently reviewing all products that include “natural flavorings” for items that are unacceptable. All new vendors are required to sign a certification letter clearly stating -

Vegan Protein Blend Ve Gan Pro Tein Blend
VEGAN PROTEIN BLEND FDA cGMP Guaranteed, Distributed by: Our unique 100% plant-based MCT Foods, LLC 630 Vernon Ave. blend is a delicious, non-GMO, Glencoe, IL 60022 high-fiber functional food that 847-835-0500 fax: 847-835-1190 is free of gluten, dairy, lactose, [email protected] soy, and corn. Orders may be placed at www.mctLean.com Discussion Clinical Applications Vegan Protein Blend is MCT Lean’s proprietary blend of • Contains 20g of protein pea protein isolate and rice protein concentrate, L-glutamine, • Contains MCTs that aid in weight loss & glycine, taurine, and SGS™ broccoli seed extract. This muscle maintenance broccoli seed extract is a super-vegetable boasting the highest level of glucoraphanin - enhancing cell detoxification • Only 6 grams net carbs per serving through free radical elimination. Our blend also contains (Cocoa flavor. Vanilla has 7g) Aminogen®, a patented, natural, plant-derived enzyme system • Supports the following: clinically proven to increase protein digestion and amino Lean body composition acid absorption - boosting nitrogen retention, aiding in the Immune health synthesis of muscle mass, and promoting deep muscle recovery. Cardiovascular health Healthy blood insulin/glucose levels Pea protein isolate features a well-balanced amino acid Gastrointestinal health BLEND PROTEIN VEGAN profile, including the highest lysine, arginine, and branched- chain amino acid (BCAA) content of all commercially available plant-based protein sources. Lysine speeds up muscle recovery time, while also playing a key role in muscle building and nitrogen level preservation. Arginine increases blood flow to allow muscles to receive nutrients and oxygen faster, thus promoting fat loss, encouraging lean muscle growth and development, and improving muscle recovery. -

Nemomex Nematicide SAFETY DATA SHEET
NemOmex Nematicide SAFETY DATA SHEET Section 1: Identification of the substance/mixture and of the company/undertaking 1.1.1.2. 1.1. Product name : NemOmex Nematicide Identification : EPA Reg. No. 82572-1-86868 CAS number : 68990-67-0 EINECS number : 273-620-4 USA (FDA) : E999 21 CFR 172.510. FEMA GRAS NUMBER 2973. 1.3.1.4. 1.2. Relevant identified uses of substance or mixture and uses advised against. Wetting agent. 1.5.1.6. 1.3. Details of supplier for the safety data sheet. Omex Agrifluids, Inc. 1675 Dockery Avenue Selma, CA 93662 1.7.1.8. 1.4. Emergency telephone number. 1-800-424-9300 Section 2: Hazards identification 2.1. Product definition: Quillaja saponaria wood extract liquid. 2.2. Classification of the substance or mixture. According to Directive criteria, 1999/45/EC and 67/548/EEC the following amendments thereof: Xi : Irritant. R Phases : R36 Irritating to eyes. S Phrases : S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Regulation criteria 1272/2008 (CLP/GHS): : warning. Hazard Statements : H320. Causes eye irritation. Precautionary Statements: P264 : Wash with water the parts of your body that had contact with the product. P280 : Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 : IF IN EYES. Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do so, continue rinsing. P314 : Get medical advice/attention if you feel unwell. P362 : Take off contaminated clothing and wash before reuse. Page 1 of 9 NemOmex Nematicide P501 : Dispose of contents/container in accordance with applicable regulations. -

Contributions to the Solution of Phylogenetic Problem in Fabales
Research Article Bartın University International Journal of Natural and Applied Sciences Araştırma Makalesi JONAS, 2(2): 195-206 e-ISSN: 2667-5048 31 Aralık/December, 2019 CONTRIBUTIONS TO THE SOLUTION OF PHYLOGENETIC PROBLEM IN FABALES Deniz Aygören Uluer1*, Rahma Alshamrani 2 1 Ahi Evran University, Cicekdagi Vocational College, Department of Plant and Animal Production, 40700 Cicekdagi, KIRŞEHIR 2 King Abdulaziz University, Department of Biological Sciences, 21589, JEDDAH Abstract Fabales is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae), Polygalaceae, Surianaceae and Quillajaceae. The monophyly of the order is supported strongly by several studies, although interfamilial relationships are still poorly resolved and vary between studies; a situation common in higher level phylogenetic studies of ancient, rapid radiations. In this study, we carried out simulation analyses with previously published matK and rbcL regions. The results of our simulation analyses have shown that Fabales phylogeny can be solved and the 5,000 bp fast-evolving data type may be sufficient to resolve the Fabales phylogeny question. In our simulation analyses, while support increased as the sequence length did (up until a certain point), resolution showed mixed results. Interestingly, the accuracy of the phylogenetic trees did not improve with the increase in sequence length. Therefore, this study sounds a note of caution, with respect to interpreting the results of the “more data” approach, because the results have shown that large datasets can easily support an arbitrary root of Fabales. Keywords: Data type, Fabales, phylogeny, sequence length, simulation. 1. Introduction Fabales Bromhead is a cosmopolitan angiosperm order which consists of four families, Leguminosae (Fabaceae) Juss., Polygalaceae Hoffmanns. -

Recruitment Dynamics of the Relict Palm, Jubaea Chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile
RESEARCH ARTICLE Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile Marina Fleury1,2*, Wara Marcelo2¤, Rodrigo A. Vásquez2, Luis Alberto González1, Ramiro O. Bustamante2 1 Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, Santiago, Chile, 2 Instituto de Ecología y Biodiversidad, Departamento de Ciencias Ecológicas, Universidad de Chile, Santiago, Chile ¤ Current address: Instituto de Conservación, Biodiversidad y Territorio, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Valdivia, Chile * [email protected] OPEN ACCESS Citation: Fleury M, Marcelo W, Vásquez RA, Abstract González LA, Bustamante RO (2015) Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Shrubs can have a net positive effect on the recruitment of other species, especially relict Intricate and Pervasive Effects of Invasive Herbivores species in dry-stressful conditions. We tested the effects of nurse shrubs and herbivory and Nurse Shrubs in Central Chile. PLoS ONE 10(7): defoliation on performance (survival and growth) of nursery-grown seedlings of the largest e0133559. doi:10.1371/journal.pone.0133559 living palm, the relict wine palm Jubaea chilensis. During an 18-month period, a total of Editor: Jin-Song Zhang, Institute of Genetics and more than 300 seedlings were exposed to of four possible scenarios produced by indepen- Developmental Biology, Chinese Academy of Sciences, CHINA dently weakening the effects of nurse shrubs and browsers. The experiment followed a two- way fully factorial design. We found consistent differences in survival between protected Received: March 2, 2015 and unprotected seedlings (27.5% and 0.7%, respectively), and herbivory had a dramatic Accepted: June 28, 2015 and overwhelmingly negative effect on seedling survival. -

TOTAL FOLATE in PEANUTS and PEANUT PRODUCTS by LAKSHMI
TOTAL FOLATE IN PEANUTS AND PEANUT PRODUCTS by LAKSHMI KOTA (Under the Direction of Ronald R. Eitenmiller) ABSTRACT Samples consisting of 222 cultivar specific samples of four different peanut types (Runner, Virginia, Spanish and Valencia) from 2 years (2005 and 2006) from 3 geographical locations (Southeast, Southwest and Virginia/Carolina) were collected for the study. No significant differences were noted among the folate levels by types for 2005 crop year peanuts (P>0.05). For 2006 peanuts, Spanish peanuts were statistically lower in folate than Runner and Virginia peanuts (P<0.05). For the Runner cultivars, significant (P<0.05) differences existed among cultivars with some significant year-to-year variation. Year of harvest did not have significant effect on folate content of Virginia peanuts. Folate contents among Virginia cultivars were statistically similar in 2005 and 2006. For Spanish cultivars in 2005, OLin had significantly higher folate than Tamspan 90. Overall means for both Runner and Spanish peanut types showed that high-oleic cultivars contained significantly higher total folate levels than normal cultivars (P<0.05). Folate levels in peanuts from Virginia/Carolina region varied significantly by production year, but peanuts from Southeast and Southwest region did not vary from 2005 to 2006. Response surface methodology (RSM) was used to optimize the trienzyme digestion for the extraction of total folate from peanut butter. The predicted second-order polynomial model was adequate (R 2 = 0.97) with a small coefficient of variation (3.05). Both Pronase R and conjugase had significant effects on the extraction. Ridge analysis gave an optimum trienzyme time: Pronase R, 1h; α-amylase, 1.5 h; conjugase, 1h. -
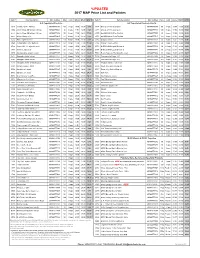
*UPDATED 2017 MAP Price List and Policies
*UPDATED 2017 MAP Price List and Policies Item # Item Description UPC number Size Form Whsle MSRP MAP $ Item # Item Description UPC number Size Form whsle MSRP MAP $ A-Z Capsulated Products A-Z Capsulated Products Cont'd. A715 Acai Energy 4:1 400 mg 601944777159 60 Vcaps 10.00 19.99 9.99 O734 Olive Leaf 18% Oleuropein 601944777340 60 Vcaps 10.00 19.99 9.99 A704 Activin Grape SE w/Amla 125 mg 601944777043 30 Vcaps 7.50 14.99 7.49 O773 Olive Leaf 18% Oleuropein 601944777739 120 Vcaps 17.50 34.99 17.49 A723 Activin Grape SE w/Amla 125 mg 601944777234 90 Vcaps 17.50 34.99 17.49 O750 OptiMSM 99.9% Pure Distilled 601944777500 90 Vcaps 10.00 19.99 9.99 A841 African Mango 10:1 601944778415 60 Vcaps 10.00 19.99 9.99 O751 OptiMSM 99.9% Pure Distilled 601944777517 180 Vcaps 20.00 39.99 19.99 A745 Amla, organic extract 601944777456 60 Vcaps 10.00 19.99 9.99 O765 Oregano extract 601944777654 60 Vcaps 8.50 16.99 8.49 A747 Andrographis, with Elderberry 601944777470 60 Vcaps 12.50 24.99 12.49 P766 Passion Flower extract 601944777661 60 Vcaps 15.00 29.99 14.99 A788 Arjuna 10:1, 1% arjunolic acids 601944777883 60 Vcaps 10.00 19.99 9.99 Q753 QVEG CoQ10 Liquid Enhanced 601944777531 30 LVcaps 17.96 29.99 14.99 A733 Artichoke, European 601944777333 60 Vcaps 17.50 34.99 17.49 Q754 QVEG CoQ10 Liquid Enhanced 601944777548 60 LVcaps 32.93 54.99 27.49 A735 Ashwagandha, organic extract 601944777357 60 Vcaps 12.50 24.99 12.49 R743 Reishi, Supreme Red Ling Zhi extract 601944777432 60 Vcaps 15.00 29.99 14.99 A727 Astragalus herbal extract 601944777272 60 Vcaps 10.00 -

GRAS Notice 851, Pea Protein
GRAS Notice (GRN) No. 851 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory 1001 G Street, N.W. Suite 500 West Washington, D.C. 20001 tel. 202.434.4100 fax 202.434.4646 Writer's Direct Access Evangelia C. Pelonis (202) 434-4 l06 pe I on i s@k h I aw. com January 28, 2019 Via FedEx & CD-ROM . Dr. Susan Carlson Director, Division of Biotechnology and GRAS Notice Review Office of Food Additive Safety (HFS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740-3835 Re: GRAS Notification for Roquette Freres Pea Protein Isolate Dear Dr. Carlson: We respectfully submit the attached GRAS Notification on behalf of our client, Roquette Freres (Roquette) for pea protein isolate to be used as a concentrated, highly digestible protein source in various food categories (excluding infant formula) and as a binder and extender in meat and poultry products. The pea protein isolate will be used as a substitute for, and/or in conjunction with, other proteins in conventional food products, as well as in meal replacement and dry blend protein powder applications. Thus, the pea protein isolate will not contribute any additional exposure to protein and it is not intended to be used to replace the entire daily protein intake or as the sole source of protein in the diet for consumers. More detailed information regarding product identification, intended use levels, and the manufacturing and safety of the ingredient is set forth in the attached GRAS Notification.