GRAS Notice 851, Pea Protein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Profile and Functional Properties of Seed Proteins from Six Pea (Pisum Sativum) Genotypes
Int. J. Mol. Sci. 2010, 11, 4973-4990; doi:10.3390/ijms11124973 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes Miroljub Barać 1,*, Slavica Čabrilo 2, Mirjana Pešić 1, Sladjana Stanojević 1, Sladjana Žilić 3, Ognjen Maćej 1 and Nikola Ristić 1 1 Faculty of Agriculture, University of Belgrade, Nemanjina 6, 11000 Belgrade-Zemun, Serbia; E-Mails: [email protected] (M.P.); [email protected] (S.S.); [email protected] (O.M.); [email protected] (N.R.) 2 High technical School, Požarevac, Serbia; E-Mails: [email protected] 3 Maize Research Institute, “Zemun Polje”, Slobodana Bajića 1, 11000 Belgrade-Zemun, Serbia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +381-11-36-15-315; Fax: +381-11-21-99-711. Received: 5 October 2010; in revised form: 21 October 2010 / Accepted: 16 November 2010 / Published: 3 December 2010 Abstract: Extractability, extractable protein compositions, technological-functional properties of pea (Pisum sativum) proteins from six genotypes grown in Serbia were investigated. Also, the relationship between these characteristics was presented. Investigated genotypes showed significant differences in storage protein content, composition and extractability. The ratio of vicilin:legumin concentrations, as well as the ratio of vicilin + convicilin: Legumin concentrations were positively correlated with extractability. Our data suggest that the higher level of vicilin and/or a lower level of legumin have a positive influence on protein extractability. -

Legumin Svnthesis in Developing Cotyledons of Vicia.Faba L.1 Received for Publication March 9,1971
Plant Physiol. (1971) 48, 419-425 Legumin Svnthesis in Developing Cotyledons of Vicia.faba L.1 Received for publication March 9,1971 ADELE MILLERD,2 M. SIMON, AND H. STERN Department of Biology, University of California, San Diego, La Jolla, California, 92037 ABSTRACT discarded. The seed coats were removed and the plant axes, which were well defined, were removed by dissection. Downloaded from https://academic.oup.com/plphys/article/48/4/419/6091415 by guest on 24 September 2021 The synthesis of legumin in developing cotyledons of Vicia Isolation of Legumin and Vicilin. Cotyledons were blended faba L. has been examined as a potential system for approach- with 2 volumes (w/v) 50 mm tris buffer, pH 7.8, containing ing the problem of differential gene expression. The pattern 0.2 M NaCl (NaCl-buffer), and the resulting brei was stirred of legumin synthesis was determined during the growth of the at 4 C for 1 hr. The brei was filtered through Miracloth, and cotyledon by microcomplement fixation which provided a the filtrate was centrifuged at 4000g for 10 min. The super- sensitive and specific assay for legumin in the presence of natant was brought to 40% saturation with (NH4)S04, and the vicilin. Legumin was detected even in young cotyledons. How- precipitate was discarded. The supernatant was brought to ever, when the cotyledons were about 10 millimeters long, and 70% saturation with (NH,)1S04. The resulting precipitate was cell division was essentially complete, there was a sharp in- dissolved in 10 mm tris buffer, pH 7.8, and refractionated crease in the rate of legumin accumulation. -

Full Pleasure with No Added Sugars with Sandwich Cookie
No added sugars sandwich cookie BAKING SOLUTIONS full pleasure with no added sugars N S O R A GA DDED SU GREAT TASTE EXPERIENCE NICE BITE Only for reference, a real product could be seen different. product a real Only for reference, No added sugars sandwich cookie full pleasure with no added sugars BAKING SOLUTIONS KEY FACTS THE RECIPE (NO ADDED SUGARS SANDWICH BISCUIT) SweetPearl® LIST OF INGREDIENTS (Detailed recipe: LBAKCOO015) The sugar-like solution that helps you combine nutrition with indulgence. BISCUIT: FILLING: • Flour • SweetPearl® P130 • A sweet bulk sweetner produced • SweetPearl® P90 • Vegetable fat from maize or wheat • Vegetable fat • Whole milk powder •Easy to use • Water • Soy lecithin • LYCASIN® 75/75 • Flavor • Kosher & Halal certified • Skimmed milk powder Baking powder •Non GMO • • Salt • Soy lecithin SweetPearl® KEY BENEFITS FOR SANDWICH COOKIE Nutritional • Sugars-free and low calorie value: 2.4 kcal/g • Low Glycaemic response (GR: 29) • Good digestive tolerance Functional • Best sugar replacer • Good functionality: behaves like sugar : melts during cooking and recrystallizes • Ease of use: 1 to 1 susbtitution • Range of particle sizes adapted to all the applications Sensory • Sweet taste : sweetening index: 80% sucrose • Taste profile similar to the one of sucrose (no aftertaste) SUGGESTED CLAIMS* NUTRITION FACTS (EUROPE) SERVING SIZE: 23g of sandwich cookie (control) Calories Protein Carb 14.8g Fiber Fat • With no added sugar 112kcal 1.5g of which sugars: 7.6g 0.4g 5.1g (contains naturally occuring sugars) * Information based on EU regulations. SERVING SIZE: 23g of no added sugars sandwich cookie (2 biscuits) Subject to applicable local laws and regulations. -

Clean Lean Protein Technical Bulletin
SEPT 2015 CLEAN LEAN PROTEIN TECHNICAL BULLETIN ULTRA-HIGH BIOLOGICAL VALUE GOLDEN PEA PROTEIN, ALKALINE, NO ADDED MALTODEXTRIN, LACTOSE, POLYOLS OR FODMAPS All 9 essential amino acids. High glutamine. Very low carbs and low fat. No fillers, no artificial additives, preservatives, sweeteners, flavours or colours DISCLAIMER For educational and informational use by practitioners and businesses only The information in this Technical Bulletin is intended exclusively for business- to-business use, and not for the end consumer. The Technical Bulletin has been compiled independently by ANH Consultancy Ltd (The Atrium, Curtis Road, Dorking, Surrey RH4 1XA, UK) to provide education and information specifically for qualified healthcare practitioners, retailers and sport and fitness professionals with interests in the NuZest product range or ingredients contained within it. Accordingly, NuZest can accept no responsibility for the quality or accuracy of the material contained herein. The information and any implied health claims made are not necessarily authorised by national or EU authorities for communication to the end consumer. GLUTEN FREE • DAIRY FREE • LECTIN FREE • SOY FREE • GMO FREE T 1300 575 121 W nuzest.com.au E [email protected] facebook.com/nuzest.aus NUZEST CLEAN LEAN PROTEIN CONTENTS 1 INTRODUCTION & PRODUCT JUSTIFICATION 2 PEA PROTEIN ISOLATE 3 NUTRITIONAL PROFILE 4 MANAGING THE BODY’S AMINO ACID POOL 6 SUPPORTING ATHLETES & ACTIVE LIFESTYLES 8 WHAT’S IN CLEAN LEAN PROTEIN? 10 CLEAN LEAN PROTEIN 11 REFERENCES 12 T 1300 575 121 W nuzest.com.au E [email protected] facebook.com/nuzest.aus INTRODUCTION & PRODUCT JUSTIFICATION 2 NUZEST’S CLEAN LEAN PROTEIN (CLP) IS A UNIQUE, VEGETARIAN AND VEGAN, PROTEIN CHARACTERISED BY ITS TASTE (4 NATURAL FLAVOURS AND AN UNFLAVOURED), LACK OF FILLERS OR FOOD ADDITIVES, AND VERY HIGH DIGESTIBILITY. -

Europe's High Technology Harvest
Ref. Ares(2014)78162 - 15/01/2014 THE COMPETITIVENESS OF THE STARCH INDUSTRY AAF Association des Amidonniers et Féculiers The European Starch Industry Association 15-16 November 2007 1 I. The European Starch Industry II. Issues impacting our competitiveness III. Future outlook for the industry 2 I. The European Starch Industry 3 Membership 24 companies - 6 associate members • Agrana Stärke • Remy Industries • AKV Langholt • Roquette Frères • Altia Corporation • Skrobarny Pelhrimov • Avebe Group • Südstärke • Cargill • Sveriges Stärkelseproducenter Förening - • Chamtor Lyckeby Stärkelsen • Copam • Syral • Crespel & Deiters • Tate & Lyle • Emsland Stärke • Wielkopolskie Przedsiebiorstwo • Finnamyl Przemyslu Ziemniaczanego (WPPZ) • Finnsugar Associate Members • Hermann Kröner • BSIA • Hungrana • Fachverband der Stärke-Industrie • Jäckering • Finnish Starch Manufacturers Association • Kartoffelmelcentralen (KMC) • Humaiz • National Starch & Chemical • USIPA Potato starch producers • VNFG 4 AAF Members location AAF Members are located in 20 out of the 27 European countries. 5 Industry data • In 2006, the AAF members used : → Approx. 14.2 Mio tons of cereals → Approx. 7.9 Mio tons of starch potatoes ⇒ to produce 9.6 Mio tons of hunderds of different starch products, derivatives and a range of co-products. • The starch industry processes almost exclusively EU raw materials. 6 Industry data • Total number of production sites in EU 27: 77 • Total number of Direct Employees: ~ 15.500 • Total turnover (sales) in EUROS for the year 2007: ~ 7.5 bn € • Average investments from 2005 to 2007 included: ~ 1.2 bn € • Average investments per year in Research and Development from 2005 to 2007 included: ~ 100 Mio € 7 The European starch industry : an essential role in the food chain We are a main supplier of the food and drink industry which is the largest EU manufacturing sector and the largest employer. -

Nutrition & Supplements Catalog
2 019 — 2020 But wait, there’s MORE! We can’t publish all our items in one catalog—it takes SIX catalogs! Find details about the different AZURE STANDARD catalogs we offer throughout AzureStandard.com the year on page 2. Nutrition & Supplements Catalog 971-200-8350 | 971-200-8350 Nutrition & Supplements THE ITEMS IN THIS CATALOG meet a high threshold for quality. Azure requires transparency from all the companies we work with, and we only choose suppliers that produce real foods made with only natural ingredients. That means you get nutrient-rich foods that are minimally processed with no artificial additives, no preservatives, non-GMO, no MSG, no artificial colors or flavors. That doesn’t mean every item is organic. Some are made with organic ingredients but are not Certified Organic. Some go far beyond organic. They all are earth-friendly, non-GMO and meticulously chosen for their healthful qualities. Azure’s Unacceptable Ingredient List for Food Items: Artificial Colors Certified Colors Pork Products Artificial Flavors Fluoride Shellfish Products (except Artificial Nitrates/Nitrites in Genetically Modified when used as an ingredient Meat Products Organisms (GMOs) in nutritional supplements) Artificial Preservatives Monosodium Glutamate Alcohol Artificial Sweeteners (MSG) Tobacco Bleached Flour Refined Conventional Sugars A Word about “Natural” Flavorings: Azure is currently reviewing all products that include “natural flavorings” for items that are unacceptable. All new vendors are required to sign a certification letter clearly stating -

Vegan Protein Blend Ve Gan Pro Tein Blend
VEGAN PROTEIN BLEND FDA cGMP Guaranteed, Distributed by: Our unique 100% plant-based MCT Foods, LLC 630 Vernon Ave. blend is a delicious, non-GMO, Glencoe, IL 60022 high-fiber functional food that 847-835-0500 fax: 847-835-1190 is free of gluten, dairy, lactose, [email protected] soy, and corn. Orders may be placed at www.mctLean.com Discussion Clinical Applications Vegan Protein Blend is MCT Lean’s proprietary blend of • Contains 20g of protein pea protein isolate and rice protein concentrate, L-glutamine, • Contains MCTs that aid in weight loss & glycine, taurine, and SGS™ broccoli seed extract. This muscle maintenance broccoli seed extract is a super-vegetable boasting the highest level of glucoraphanin - enhancing cell detoxification • Only 6 grams net carbs per serving through free radical elimination. Our blend also contains (Cocoa flavor. Vanilla has 7g) Aminogen®, a patented, natural, plant-derived enzyme system • Supports the following: clinically proven to increase protein digestion and amino Lean body composition acid absorption - boosting nitrogen retention, aiding in the Immune health synthesis of muscle mass, and promoting deep muscle recovery. Cardiovascular health Healthy blood insulin/glucose levels Pea protein isolate features a well-balanced amino acid Gastrointestinal health BLEND PROTEIN VEGAN profile, including the highest lysine, arginine, and branched- chain amino acid (BCAA) content of all commercially available plant-based protein sources. Lysine speeds up muscle recovery time, while also playing a key role in muscle building and nitrogen level preservation. Arginine increases blood flow to allow muscles to receive nutrients and oxygen faster, thus promoting fat loss, encouraging lean muscle growth and development, and improving muscle recovery. -
Addressing Sugar Reduction Demands
ADDRESSING Why should we consider SUGARS reducing sugars? REDUCTION Consumers’ perception Public health Regulatory DEMANDS and expectations factors pressure CONSUMERS’ PERCEPTION AND EXPECTATIONS Consumers look to a healthier lifestyle Top of mind in terms of health for consumers. Key priority for the industry in terms of finding SUGARS in U.S. consumers would rather cut back on effective formulation solutions. 3 5 Source: Innova Market Insights, What’s your sugar strategy?, June 2020 sugars than consume artificial sweeteners. PUBLIC HEALTH FACTORS Overweight and Obese Oral diseases and EXCESS OF ADDED tooth decay 1.9 billion overweight globally¹ SUGARS INTAKE • incl. 650 million adults obese CAN LEAD TO: • i.e. 13% of the world’s adult population A risk of non-communicable Worldwide prevalence of obesity x3 diseases (NCDs). 3.5 1.9 from 1975 to 2016¹ A higher risk of developing billion billion dental caries. Diabetes Cardiovascular 3.5 billion people worldwide affected by oral diseases² 422 million adults with diabetes diseases globally¹ • 1.6 million deaths from diabetes #1 cause of death globally¹ • 2.2 million deaths from high blood glucose Decays of permanent teeth: 2.3 422 #1 17.9 million people died from CVDs billion people globally² million Worldwide prevalence of diabetes cause of death i.e. 31% of all global deaths¹ Decays of primary teeth: 530 x4 from 1980 to 2014¹ million children globally² ¹ World Health Statistics 2016, World Health Organization, global statistics - adults aged 18 years and older, 2016 ² Global Burden of Disease -

TOTAL FOLATE in PEANUTS and PEANUT PRODUCTS by LAKSHMI
TOTAL FOLATE IN PEANUTS AND PEANUT PRODUCTS by LAKSHMI KOTA (Under the Direction of Ronald R. Eitenmiller) ABSTRACT Samples consisting of 222 cultivar specific samples of four different peanut types (Runner, Virginia, Spanish and Valencia) from 2 years (2005 and 2006) from 3 geographical locations (Southeast, Southwest and Virginia/Carolina) were collected for the study. No significant differences were noted among the folate levels by types for 2005 crop year peanuts (P>0.05). For 2006 peanuts, Spanish peanuts were statistically lower in folate than Runner and Virginia peanuts (P<0.05). For the Runner cultivars, significant (P<0.05) differences existed among cultivars with some significant year-to-year variation. Year of harvest did not have significant effect on folate content of Virginia peanuts. Folate contents among Virginia cultivars were statistically similar in 2005 and 2006. For Spanish cultivars in 2005, OLin had significantly higher folate than Tamspan 90. Overall means for both Runner and Spanish peanut types showed that high-oleic cultivars contained significantly higher total folate levels than normal cultivars (P<0.05). Folate levels in peanuts from Virginia/Carolina region varied significantly by production year, but peanuts from Southeast and Southwest region did not vary from 2005 to 2006. Response surface methodology (RSM) was used to optimize the trienzyme digestion for the extraction of total folate from peanut butter. The predicted second-order polynomial model was adequate (R 2 = 0.97) with a small coefficient of variation (3.05). Both Pronase R and conjugase had significant effects on the extraction. Ridge analysis gave an optimum trienzyme time: Pronase R, 1h; α-amylase, 1.5 h; conjugase, 1h. -
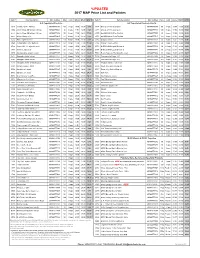
*UPDATED 2017 MAP Price List and Policies
*UPDATED 2017 MAP Price List and Policies Item # Item Description UPC number Size Form Whsle MSRP MAP $ Item # Item Description UPC number Size Form whsle MSRP MAP $ A-Z Capsulated Products A-Z Capsulated Products Cont'd. A715 Acai Energy 4:1 400 mg 601944777159 60 Vcaps 10.00 19.99 9.99 O734 Olive Leaf 18% Oleuropein 601944777340 60 Vcaps 10.00 19.99 9.99 A704 Activin Grape SE w/Amla 125 mg 601944777043 30 Vcaps 7.50 14.99 7.49 O773 Olive Leaf 18% Oleuropein 601944777739 120 Vcaps 17.50 34.99 17.49 A723 Activin Grape SE w/Amla 125 mg 601944777234 90 Vcaps 17.50 34.99 17.49 O750 OptiMSM 99.9% Pure Distilled 601944777500 90 Vcaps 10.00 19.99 9.99 A841 African Mango 10:1 601944778415 60 Vcaps 10.00 19.99 9.99 O751 OptiMSM 99.9% Pure Distilled 601944777517 180 Vcaps 20.00 39.99 19.99 A745 Amla, organic extract 601944777456 60 Vcaps 10.00 19.99 9.99 O765 Oregano extract 601944777654 60 Vcaps 8.50 16.99 8.49 A747 Andrographis, with Elderberry 601944777470 60 Vcaps 12.50 24.99 12.49 P766 Passion Flower extract 601944777661 60 Vcaps 15.00 29.99 14.99 A788 Arjuna 10:1, 1% arjunolic acids 601944777883 60 Vcaps 10.00 19.99 9.99 Q753 QVEG CoQ10 Liquid Enhanced 601944777531 30 LVcaps 17.96 29.99 14.99 A733 Artichoke, European 601944777333 60 Vcaps 17.50 34.99 17.49 Q754 QVEG CoQ10 Liquid Enhanced 601944777548 60 LVcaps 32.93 54.99 27.49 A735 Ashwagandha, organic extract 601944777357 60 Vcaps 12.50 24.99 12.49 R743 Reishi, Supreme Red Ling Zhi extract 601944777432 60 Vcaps 15.00 29.99 14.99 A727 Astragalus herbal extract 601944777272 60 Vcaps 10.00 -

Filler and Filler-Binder Solutions an Unwavering Commitment to Enabling Life-Saving Pharmaceuticals Fillers and Filler-Binders: a Balancing Act
FILLER AND FILLER-BINDER SOLUTIONS AN UNWAVERING COMMITMENT TO ENABLING LIFE-SAVING PHARMACEUTICALS FILLERS AND FILLER-BINDERS: A BALANCING ACT The ideal formulation delivers a defect-free dosage form in the widest range of manufacturing conditions Enable effective drug manufacturing possible, which is obtained by balancing the brittle, plastic and elastic behavior exhibited by the deformation of the excipients and API under compaction. and enhance patient compliance Plastic behavior • Fewer surfaces generated during compaction, which makes ingredients sensitive to lubrication with our expansive range of reliable • Higher mechanical strength • Higher propensity to cap, higher sensitivity to turret speed fillers and filler-binders. Compressive forces Plastic behavior Final dosage forms come in all shapes and sizes, and our focus is providing formulators and Elastic recovery manufacturers with flexible, high-quality ingredients that enable cost-effective production. No matter your formulation, dosage or process constraints, Roquette offers a diverse range of adaptable filler and filler-binder products and the technical expertise to ensure the right excipient solution is selected. Our commitment to consistency is what sets us apart. Each of our excipients is manufactured Fragmentation behavior under Roquette quality control systems, ensuring consistent product performance for optimum • Fragmentation under compression generates new surfaces fresh of lubricants, making ingredients productivity. From laboratory to industrial scale production, -

Plaster Industry
ROQUETTE WORLDWIDE EEUROPEU R O P E TURKEY Roquette Tarım ve Gıda FRANCE San. ve Tic. Ltd. Sti. From Do-it-yourself Roquette Frères Büyükdere Cad. Harman Sok. · Corporate Headquarters Duran Iş merkezi No:4 K:3 · 62080 Lestrem cedex - France 34394 Levent Istanbul - Turkey to industrial applications, Telephone: + 33 3 21 63 36 00 Telephone: + 90 212 234 83 73 Fax: + 33 3 21 63 38 50 Fax: + 90 212 234 83 74 BELGIUM our solutions J.V Roquette & Barentz AAMERICAM E R I C A Excelsiorlaan 7 Box 2 USA 1930 Zaventem - Belgium Roquette America Inc. Telephone: + + 00 32 2 714 13 00 are available for Geneva Innovation Center DENMARK 2211 Innovation Drive Roquette ApS Geneva, IL 60134 - USA Telephone: + 1 630 463 9430 Gydevang 39-41 Fax: + 1 630 232 2157 DK - 3450 Allerød Telephone: + 45 69 663 200 MEXICO Fax: + 45 69 663 209 Roquette México S.A. de C.V Blvd. Bernardo Quintana 9750 of 321 FINLAND Fracc. Centro sur Queretaro Qro. Roquette Nordica Oy CP 76090 - México Ahventie 4A, 20 Telephone: + 52 44 2229 1270 Solutions FI-02170 Espoo – Finland Fax: + 52 44 2229 1270 Telephone: + 358 9 315 85 700 Fax: + 358 9 8632 113 ASIAA S I A for the GERMANY CHINA Roquette GmbH Roquette Shanghai Darmstädter Landstrasse 182 Room 505— K. Wah Centre, 60598 Frankfurt - Deutschland 1010 Huai Hai Zhong Road, Telephone: + 49 69 60 91 050 Shanghai 200031 - P.R. China r Fax: + 49 69 60 91 05 59 Telephone: + 86 21 54 03 99 22 ste Fax: + 86 21 54 03 66 06 a ITALY l Ready- PPlaster Roquette Italia S.p.A INDIA Tiles Via Serravalle 26 Roquette India Private Limited to-use Plasters Molding 15063 Cassano Spinola Office n° 702, 7th Floor Powai Plaza ds plasters ry Alessandria - Italia Hiranandani Gardens - Powai dry blends st Telephone: + 39 0 143 7741 Mumbai 400 076 - India du Fax: + 39 0 143 477 295 Telephone: + 91 22 2570 6775 iindustryn Fax: + 91 22 2570 6770 ROMANIA Roquette Romania S.A.