Subchapter B—Food for Human Consumption (Continued)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States July 2016 2 Table of Contents
Deuterium Labelled Compounds United States July 2016 2 Table of Contents International Distributors 3 Corporate Overview 4 General Information 5 Pricing and Payment 5 Quotations 5 Custom Synthesis 5 Shipping 5 Quality Control 6 Quotations 6 Custom Synthesis 6 Shipping 6 Quality Control 6 Chemical Abstract Service Numbers 6 Handling Hazardous Compounds 6 Our Products are Not Intended for Use in Humans 7 Limited Warranty 7 Packaging Information 7 Alphabetical Listings 8 Stock Clearance 236 Products by Category 242 n-Alkanes 243 α-Amino Acids, N-Acyl α-Amino Acids, N-t-BOC Protected α-Amino Acid 243 and N-FMOC Protected α-Amino Acids Buffers and Reagents for NMR Studies 245 Detergents 245 Environmental Standards 246 Fatty Acids and Fatty Acid Esters 249 Flavours and Fragrances 250 Gases 253 Medical Research Products 254 Nucleic Acid Bases and Nucleosides 255 Pesticides and Pesticide Metabolites 256 Pharmaceutical Standards 257 Polyaromatic Hydrocarbons (PAHs), Alkyl-PAHs, Amino-PAHs, 260 Hydroxy-PAHs and Nitro-PAHs Polychlorinated Biphenyls (PCBs) 260 Spin Labels 261 Steroids 261 3 International Distributors C Beijng Zhenxiang H EQ Laboratories GmbH Australia K Technology Company Graf-von-Seyssel-Str. 10 Rm. 15A01, Changyin Bld. 86199 Augsburg Austria H No. 88, YongDingLu Rd. Germany Beijing 100039 Tel.: (49) 821 71058246 Belgium J China Fax: (49) 821 71058247 Tel.: (86) 10-58896805 [email protected] China C Fax: (86) 10-58896158 www.eqlabs.de Czech Republic H [email protected] Germany, Austria, China Czech Republic, Greece, Denmark I Hungary, -

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

GRAS Notice (GRN) No. 903, Quillaia Extract Type 2
GRAS Notice (GRN) No. 903 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory NATUREX• part of Givaudan 'fR1@CG~nill~(jJ) 07 January 2020 JAN 1 5 2020 Dr. Paulette Gaynor FOOD OF, ,Li,; UF L ADDITIVE SAFETY Office of Food Additive Safety (HFS-200) __J Center for Food Safety and Applied Nutrition (CFSAN) Food and Drug Administration 5001 Campus Drive College Park, MD 20740 USA Dear Dr. Gaynor: Re: GRAS Notice for Qulllaia Extract Type 2 In accordance with 21 CFR §170 Subpart E consisting of§§ 170.203 through 170.285, Naturex SA (250 rue Pierre Bayle, BP 81218 - 84911, Avignon, Cedex 9, France], as the notifier, is submitting one hard copy and one electronic copy (on CD) of the GRAS Notice for quillaia extract type 2, containing all data and lnfonnation supporting the company's conclusion that quillaia extract type 2 is GRAS on the basis of scientific procedures, for use in specified conventional food and beverage products across multiple categories; these food uses of quillaia extract type 2 are therefore not subject to the premarket approval requirements of the Federal Food, Drug and Cosmetic Act. Information setting forth the basis for Naturex's GRAS conclusion, as well as a consensus opinion of an independent panel of experts, also are enclosed for review by the agency. I certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using Symantec Endpoint Protection 12.1.4. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. -

Cyanogenic Glycosides • Isothiocyanate Glycosides • Phenolic & Aldehyde Glycosides • Coumarins - Bitter Principles • Terpenes and Terpenoids • Tannins Glycosides
Dr. Pran Kishore Deb and Dr. Yousef Abusamra Phytotherapy • Phytotherapy is the study of the use of extracts of natural origin as medicines or health-promoting agents. • Traditional phytotherapy is a synonym for herbalism and regarded as alternative medicine by much of Western medicine. PART – 1 • Glycosides • Saponin glycosides • Flavonoid glycosides • Anthocyanidin • Cyanogenic glycosides • Isothiocyanate glycosides • Phenolic & Aldehyde glycosides • Coumarins - Bitter principles • Terpenes and Terpenoids • Tannins Glycosides • They are compounds containing a carbohydrate (sugar) and a noncarbohydrate residue in the same molecule. • The carbohydrate residue is attached by an acetal linkage at carbon atom 1 to a noncarbohydrate residue. • The carbohydrate (sugar) component is called the GLYCONE. • The noncarbohydrate component is known as the AGLYCONE. • The sugar moiety can be joined to the aglycone in various ways: 1. Oxygen (O-glycoside) 2. Sulphur (S-glycoside) 3. Nitrogen (N-glycoside) 4. Carbon (C-glycoside) Classification of Glycosides • The aglycone may be methyl alcohol, glycerol, a sterol, a phenol, etc. • Based on the chemical nature of the aglycone group, glycosides can be classified as follows: 5 SAPONIN GLYCOSIDES SAPONIN GLYCOSIDES • Saponins on hydrolysis yield an aglycone known as "sapogenin". • Saponin glycosides are divided into 2 types based on the chemical structure of their aglycones (sapogenins). Classification of Saponins: According to the nature of the aglycone: 1. Neutral saponins or Steroidal saponins with spiroketal side chains. 2. Acid saponins or Triterpenoidal saponins Sug-O Sug-O Steroidal Saponins Triterpenoidal Saponins 7 Steroidal Saponins • Neutral steroidal glycosides which contain spiroketal side chain. • Two rings E and F called ketal because they are attached through two oxygen atoms and called spiral because they are not on the same level. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -
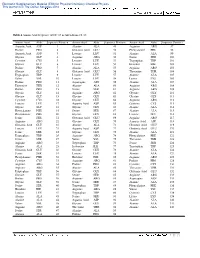
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

Japanese Flavoring Agents As Food Additives
Japanese Flavoring Agents as Food Additives In Japan, synthetic flavoring agents are allowed to be used only when they are designated by the Minister of Health, Labour and Welfare as food additives under the Japanese Food Sanitation Act. Currently, we have identified 170 chemical substances which are commonly used as flavorings as shown in Table 1. Table 2 lists 18 groups which are also from the official list of “designated additives” and contain 3004 additional flavor materials. Each of the 18 groups in Table 2 contains substances that are similar in chemical structure. For links to a complete listing of the Japanese additives used in food: (a) Designated additives, (b) Existing food additives, (c) Natural flavoring agents and (d) Ordinary foods used as food additives - go to http://www.mhlw.go.jp/english/topics/foodsafety/foodadditives/index.html Check for Flavoring updates at http://www.jffma-jp.org/english/information.html Provided with Updated Revisions (as of April 2015) By Leffingwell & Associates Table 1. Designated additives used as flavoring substances Compound Synonym or Old name CAS Acesulfame Potassium 55589-62-3 Acetaldehyde (New as of 2006.05.16) ethanal 75-07-0 Acetophenone acetophenone 98-86-2 Acetic acid, Glacial 64-19-7 Adipic Acid 124-04-9 714229-20-6 Advantame (New as of 2015.02.20) 245650-17-3 DL-Alanine 302-72-7 Allyl cyclohexylpropionate allyl cyclohexanepropionate 2705-87-5 Allyl hexanoate allyl hexanoate 123-68-2 Allyl isothiocyanate allyl isothiocyanate 57503 (3-Amino-3-carboxypropyl)dimethylsulfonium chloride -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast
FULL NUTRITIONAL CATEGORY LINKS Bagels Breads Breakfast Beverages Entrée - Bowls/Mac/Flatbread Pizza Kids Dressings Pastries & Sweets Salads Sandwiches Sides Smoothies Souffles Soups Spreads Grab N Go Catering ©2021 Panera Bread. All Rights Reserved. Bakery - Cafe Nutrition, Ingredient, and Allergen Information Effective 1/13/2021 Version 2 Bagels Asiago Cheese Bagel g) m ( l mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat (g) m i i i ASIAGO CHEESE BAGEL (ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, s ra u i ar REDUCED IRON, THIAMINE MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, ASIAGO CHEESE u (g) ole lor lor rbs (g) g d t a a h a (PASTEURIZED MILK, CHEESE CULTURE, SALT, MICROBIAL AND ANIMAL ENZYMES, POWDERED nts Servin C C Fa Sat Tran C So C Fiber (g) Su Prote CELLULOSE [TO PREVENT CAKING]), BROWN SUGAR, SALT, DOUGH IMPROVER (MALTED e i WHEAT FLOUR, ENRICHED WHEAT FLOUR [NIACIN, REDUCED IRON, THIAMINE MONONITRATE, d RIBOFLAVIN, FOLIC ACID], INACTIVATED YEAST, ACEROLA EXTRACT, FUNGAL ENZYMES), YEAST e (YEAST, SORBITAN MONOSTEARATE, ASCORBIC ACID)), ASIAGO CHEESE (PASTEURIZED MILK, 1 Bagel 320 50 5 3 0 15 530 55 2 4 14 CHEESE CULTURES, SALT, MICROBIAL & ANIMAL ENZYMES, POWDERED CELLULOSE [TO PREVENT CAKING]). Ingr CONTAINS MILK, WHEAT. Allergens * Blueberry Bagel g) m ) ( l g mg) ( Size ed Fat (g) tero (g) t g es es from Fat n (g) s s Fat ( m i i i ENRICHED FLOUR (WHEAT FLOUR, MALTED BARLEY FLOUR, NIACIN, REDUCED IRON, THIAMINE n s ra u i i ar MONONITRATE, RIBOFLAVIN, FOLIC ACID), WATER, BLUEBERRY FLAVORED -

Impact of Wort Amino Acids on Beer Flavour: a Review
fermentation Review Impact of Wort Amino Acids on Beer Flavour: A Review Inês M. Ferreira and Luís F. Guido * LAQV/REQUIMTE, Faculdade de Ciências, Universidade do Porto, Rua do Campo Alegre, 687, 4169-007 Porto, Portugal; ines.fi[email protected] * Correspondence: [email protected]; Tel.: +351-220-402-644 Received: 3 March 2018; Accepted: 25 March 2018; Published: 28 March 2018 Abstract: The process by which beer is brewed has not changed significantly since its discovery thousands of years ago. Grain is malted, dried, crushed and mixed with hot water to produce wort. Yeast is added to the sweet, viscous wort, after which fermentation occurs. The biochemical events that occur during fermentation reflect the genotype of the yeast strain used, and its phenotypic expression is influenced by the composition of the wort and the conditions established in the fermenting vessel. Although wort is complex and not completely characterized, its content in amino acids indubitably affects the production of some minor metabolic products of fermentation which contribute to the flavour of beer. These metabolic products include higher alcohols, esters, carbonyls and sulfur-containing compounds. The formation of these products is comprehensively reviewed in this paper. Furthermore, the role of amino acids in the beer flavour, in particular their relationships with flavour active compounds, is discussed in light of recent data. Keywords: amino acids; beer; flavour; higher alcohols; esters; Vicinal Diketones (VDK); sulfur compounds 1. Introduction The process by which beer has been brewed has not changed significantly since its discovery over 2000 years ago. Although industrial equipment is used for modern commercial brewing, the principles are the same. -

Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat
Pediat. Res. 6: 713-719 (1972) Amino acid neonate intestine transport, amino acid Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat J. F. FITZGERALD1431, S. REISER, AND P. A. CHRISTIANSEN Departments of Pediatrics, Medicine, and Biochemistry, and Gastrointestinal Research Laboratory, Indiana University School of Medicine and Veterans Administration Hospital, Indianapolis, Indiana, USA Extract The activity of amino acid transport pathways in the small intestine of the 2-day-old rat was investigated. Transport was determined by measuring the uptake of 1 mM con- centrations of various amino acids by intestinal segments after a 5- or 10-min incuba- tion and it was expressed as intracellular accumulation. The neutral amino acid transport pathway was well developed with intracellular accumulation values for leucine, isoleucine, valine, methionine, tryptophan, phenyl- alanine, tyrosine, and alanine ranging from 3.9-5.6 mM/5 min. The intracellular accumulation of the hydroxy-containing neutral amino acids threonine (essential) and serine (nonessential) were 2.7 mM/5 min, a value significantly lower than those of the other neutral amino acids. The accumulation of histidine was also well below the level for the other neutral amino acids (1.9 mM/5 min). The basic amino acid transport pathway was also operational with accumulation values for lysine, arginine and ornithine ranging from 1.7-2.0 mM/5 min. Accumulation of the essential amino acid lysine was not statistically different from that of nonessential ornithine. Ac- cumulation of aspartic and glutamic acid was only 0.24-0.28 mM/5 min indicating a very low activity of the acidic amino acid transport pathway.