Development of the Upper Lip
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Induction and Specification of Cranial Placodes ⁎ Gerhard Schlosser
Developmental Biology 294 (2006) 303–351 www.elsevier.com/locate/ydbio Review Induction and specification of cranial placodes ⁎ Gerhard Schlosser Brain Research Institute, AG Roth, University of Bremen, FB2, PO Box 330440, 28334 Bremen, Germany Received for publication 6 October 2005; revised 22 December 2005; accepted 23 December 2005 Available online 3 May 2006 Abstract Cranial placodes are specialized regions of the ectoderm, which give rise to various sensory ganglia and contribute to the pituitary gland and sensory organs of the vertebrate head. They include the adenohypophyseal, olfactory, lens, trigeminal, and profundal placodes, a series of epibranchial placodes, an otic placode, and a series of lateral line placodes. After a long period of neglect, recent years have seen a resurgence of interest in placode induction and specification. There is increasing evidence that all placodes despite their different developmental fates originate from a common panplacodal primordium around the neural plate. This common primordium is defined by the expression of transcription factors of the Six1/2, Six4/5, and Eya families, which later continue to be expressed in all placodes and appear to promote generic placodal properties such as proliferation, the capacity for morphogenetic movements, and neuronal differentiation. A large number of other transcription factors are expressed in subdomains of the panplacodal primordium and appear to contribute to the specification of particular subsets of placodes. This review first provides a brief overview of different cranial placodes and then synthesizes evidence for the common origin of all placodes from a panplacodal primordium. The role of various transcription factors for the development of the different placodes is addressed next, and it is discussed how individual placodes may be specified and compartmentalized within the panplacodal primordium. -
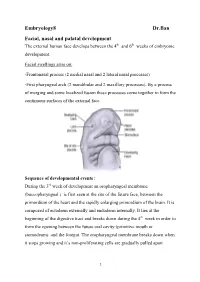
Embryology8 Dr.Ban Facial, Nasal and Palatal Development the External Human Face Develops Between the 4Th and 6Th Weeks of Embryonic Development
Embryology8 Dr.Ban Facial, nasal and palatal development The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on: -Frontonasal process (2 medial nasal and 2 lateral nasal processes) -First pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. Sequence of developmental events : During the 3rd week of development an oropharyngeal membrane (buccopharyngeal ) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing and it’s non-proliferating cells are gradually pulled apart 1 because they cannot fill the expanding area.The tissues around it expand very rapidly. The face develops from five primordia that appear in the fourth week: the frontonasal prominence, the two maxillary swellings, and the two mandibular swellings. The external face forms from two sources that surround the oropharyngeal membrane 1-Tissues of the frontonasal process that cover the forebrain, predominantly of neural crest origin. 2-The tissues of the first (or mandibular) pharyngeal arch, of mixed mesoderm and neural crest origin Face initially formed by 5 mesenchymal swellings ( prominences): Two mandibular prominences Two maxillary prominences Frontonasal prominence (midline structure is a single structure that is ventral to the forebrain. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Loss-Of-Function Mutation in the Prokineticin 2 Gene Causes
Loss-of-function mutation in the prokineticin 2 gene SEE COMMENTARY causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism Nelly Pitteloud*†, Chengkang Zhang‡, Duarte Pignatelli§, Jia-Da Li‡, Taneli Raivio*, Lindsay W. Cole*, Lacey Plummer*, Elka E. Jacobson-Dickman*, Pamela L. Mellon¶, Qun-Yong Zhou‡, and William F. Crowley, Jr.* *Reproductive Endocrine Unit, Department of Medicine and Harvard Reproductive Endocrine Science Centers, Massachusetts General Hospital, Boston, MA 02114; ‡Department of Pharmacology, University of California, Irvine, CA 92697; §Department of Endocrinology, Laboratory of Cellular and Molecular Biology, Institute of Molecular Pathology and Immunology, University of Porto, San Joa˜o Hospital, 4200-465 Porto, Portugal; and ¶Departments of Reproductive Medicine and Neurosciences, University of California at San Diego, La Jolla, CA 92093 Communicated by Patricia K. Donahoe, Massachusetts General Hospital, Boston, MA, August 14, 2007 (received for review May 8, 2007) Gonadotropin-releasing hormone (GnRH) deficiency in the human associated with KS, although no functional data on the mutant presents either as normosmic idiopathic hypogonadotropic hypo- proteins were provided (17). Herein, we demonstrate that homozy- gonadism (nIHH) or with anosmia [Kallmann syndrome (KS)]. To gous loss-of-function mutations in the PROK2 gene cause IHH in date, several loci have been identified to cause these disorders, but mice and humans. only 30% of cases exhibit mutations in known genes. Recently, murine studies have demonstrated a critical role of the prokineticin Results pathway in olfactory bulb morphogenesis and GnRH secretion. Molecular Analysis of PROK2 Gene. A homozygous single base pair Therefore, we hypothesize that mutations in prokineticin 2 deletion in exon 2 of the PROK2 gene (c.[163delA]ϩ [163delA]) (PROK2) underlie some cases of KS in humans and that animals was identified in the proband, in his brother with KS, and in his deficient in Prok2 would be hypogonadotropic. -

Anatomical Basis of Craniofacial Birth Defects
AnatomicalAnatomical BasisBasis ofof CraniofacialCraniofacial BirthBirth DefectsDefects Handout download: http://www.oucom.ohiou.edu/dbms-witmer/peds-rpac.htm LawrenceLawrence M.M. Witmer,Witmer, PhDPhD Department of Biomedical Sciences College of Osteopathic Medicine Ohio University Athens, Ohio 45701 [email protected] 18 October 2000 DevelopmentDevelopment ofof thethe FaceFace II • 5 facial primordia • Frontonasal prominence • Paired maxillary prominences • Paired mandibular prominences • Surround primordial mouth (stomodeum) • Neural crest: source for almost all connective tissues in the face • Frontonasal prominence forms forehead and nose and a short margin of mouth • Lower jaw and lip form first • Nasal placodes (and pit): surrounded by medial & lateral nasal prominences • Nasal pit remains connected to mouth • Maxillary prominences grow toward each other, pushing nasal prominences medially From Moore 1982 DevelopmentDevelopment ofof thethe FaceFace IIII • Medial nasal prominences merge with each other and with lateral nasal & maxillary prominences • Nasolacrimal groove: between lateral nasal and maxillary prominences • Becomes nasolacrimal duct • Duct forms as solid epithelial cord that later canalizes • Nasolacrimal duct atresia • Failure to completely canalize • 6% of newborns • Intermaxillary segment • Merger of medial nasal prominences • Gives rise to philtrum, premaxillary bones, primary palate From Moore 1982 SummarySummary ofof FacialFacial DevelopmentDevelopment From Moore 1982 Disruptions in the formation -

Development and Malformations of Face and Palate
Development and malformations of face and palate Compiled by András Csillag and Andrea D. Székely GERMINAL LAYER DERIVATIVES ECTODERM contributing to the formation of the face appears by the 4th week. The oropharyngeal membrane (interface between ECTODERM and ENDODERM) is located in front of the later palatine tonsils. Ectodermal structures limiting the stomodeum participate in the formation of the face, as well as of the nasal and oral cavities. MESENCHYME that fills the pharyngeal arches derives from the neural crest ECTOMESENCHYME DEVELOPMENT OF THE FACE WEEK 6 The face is formed by 5 processes approximately 24 days Frontonasal prominence (1) Maxillary prominence (2) 1st arch Mandibular prominence (2) nasal (olfactory) pits form surrounded by the medial and lateral nasal processes nasolacrimal groove separates the lateral nasal process from the maxillary process maxillary processes fuse with the medial nasal processes lateral nasal processes fuse with the maxillary processes, thus obliterating the nasolacrimal groove. DEVELOPMENT OF THE FACE 5-week embryo 6-week embryo 7-week embryo. 10-week embryo The nasal prominences are gradually Maxillary prominences have fused separated from the maxillary with the medial nasal prominences. prominence by deep furrows. CEPHALIC PRIMORDIA - PLACODES Olfactory placode Pharyngeal Stomodeum” arches Nasal placodes - thickenings of the surface ectoderm (later differentiate into the olfactory epithelium) DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 5 the placodes form the nasal pits. They further invaginate and the pits approach the primitive oral cavity. A thin oronasal membrane separates the two cavities. By its rupture the primitive choanae will form DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 8, a partition forms between the primitive nasal chambers and the oral cavity The primary palate - the anterior aspect derives from the intermaxillary segment (or median palatine process, formed by the medial nasal processes). -

A Combined Approach of Teaching Head Development Using Embryology and Comparative Anatomy
Edorium J Anat Embryo 2016;3:17–27. Danowitz et al. 17 www.edoriumjournals.com/ej/ae REVIEW ARTICLE PEER REVIEWED | OPEN ACCESS A combined approach of teaching head development using embryology and comparative anatomy Melinda Danowitz, Hong Zheng, Adriana Guigova, Nikos Solounias ABSTRACT the evolutionary changes of many structures allows for a greater understanding of the Many aspects of human head embryology reflect human embryology, and removes the need for its evolutionary development. The pharyngeal memorization of seemingly complex processes. arches, a major component of head development, A link to comparative evolutionary anatomy originally functioned in filter feeding and provides context to the purpose and morphology vascular exchange, which is why each arch of primitive structures, and clarifies several has associated vasculature and muscles. The issues in human head development. primitive tongue had few-associated muscles and was responsible for simple movements; the Keywords: Anatomy education, Embryology, Head human tongue evolved post-otic somites that and neck, Pharyngeal arches migrate to the tongue and develop the majority of the tongue musculature. These somites originate How to cite this article outside the tongue, and the motor innervation therefore differs from the general and special Danowitz M, Zheng H, Guigova A, Solounias N. A sensory innervation. In the primitive condition, combined approach of teaching head development the trapezius and sternocleidomastoid belonged using embryology and comparative anatomy. to a single muscle group that were involved in Edorium J Anat Embryo 2016;3:17–27. gill movements; they separate into two muscles with the reduction of certain skeletal elements, but retain the same innervation. -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Characterization of Epithelial Domains in The
Development 106, 493-509 (1989) 493 Printed in Great Britain © The Company of Biologists Limited 1989 Characterization of epithelial domains in the nasal passages of chick embryos: spatial and temporal mapping of a range of extracellular matrix and cell surface molecules during development of the nasal placode S. J. CROUCHER and C. TICKLE Department of Anatomy and Developmental Biology, University College & Middlesex School of Medicine, Windeyer Building, Cleveland Street, London W1P6DB, UK Summary The formation of the nasal passages involves complex development to gain insights into the origin of the morphogenesis and their lining develops a spatially epithelial lining of the nasal passages. All reagents bind ordered pattern of differentiation, with distinct domains at early stages to the thickened nasal placode and of olfactory and respiratory epithelium. Using anti- surrounding head ectoderm and then become progress- bodies to the neural cell adhesion molecule (N-CAM), ively restricted to the olfactory domain. The expression keratan sulphate and heparan sulphate proteoglycan of these characteristics appears to be modulated during (HSPG) and a panel of lectins (agglutinins of Canavalia development rather than being cell autonomous. ensiformis (ConA), Dolichos biflorus (DBA), peanut The distribution of keratan sulphate was compared (PNA), Ricinis communis (RCA1), soybean (SBA), Ulex with collagen type II in relation to the specification of the europaeus (UEA1), and wheatgerm (WGA)), we have chondrocranium. Keratan sulphate and collagen type II documented cell surface characteristics of each epithelial are only colocalized at the epithelial-mesenchymal inter- domain. Binding of antibodies to N-CAM and to keratan face during early nasal development. At later stages, sulphate, and the lectins ConA, PNA, RCA1, SBA and only collagen type II is expressed at the interface WGA marks the olfactory epithelial domain only. -

Illustrated Review of the Embryology and Development of the Facial
REVIEW ARTICLE Illustrated Review of the Embryology and Development of the Facial Region, Part 2: Late Development of the Fetal Face and Changes in the Face from the Newborn to Adulthood P.M. Som and T.P. Naidich ABSTRACT SUMMARY: The later embryogenesis of the fetal face and the alteration in the facial structure from birth to adulthood have been reviewed. Part 3 of the review will address the molecular mechanisms that are responsible for the changes described in parts 1 and 2. art 1 of this 3-part review primarily dealt with the early em- first make contact, each is completely covered by a homoge- Pbryologic development of the face and nasal cavity. Part 2 will neous epithelium. A special epithelium arises at the edge of discuss the later embryonic and fetal development of the face, and each palatal shelf, facilitating the eventual fusion of these changes in facial appearance from neonate to adulthood will be shelves. The epithelium on the nasal cavity surface of the palate reviewed. will differentiate into columnar ciliated epithelium. The epi- thelium on the oral cavity side of the palate will differentiate Formation of the Palate into stratified squamous epithelium. Between the sixth and 12th weeks, the palate is formed from 3 The 2 palatal shelves also fuse with the triangular primary pal- primordia: a midline median palatine process and paired lateral ate anteromedially to form a y-shaped fusion line. The point of palatine processes (Fig 1). In the beginning of the sixth week, fusion of the secondary palatal shelves with the primary palate is merging of the paired medial nasal processes forms the intermax- marked in the adult by the incisive foramen. -

Congenital Anomalies of the Nose
133 Congenital Anomalies of the Nose Jamie L. Funamura, MD1 Travis T. Tollefson, MD, MPH, FACS2 1 Department of Otolaryngology and Communication Enhancement, Address for correspondence Travis T. Tollefson, MD, MPH, FACS, Facial Children’s Hospital Boston, Boston, Massachusetts Plastic and Reconstructive Surgery, Department of Otolaryngology- 2 Department of Otolaryngology, University of California, Davis, Head and Neck Surgery, University of California, Davis, 2521 Stockton Sacramento, California Blvd., Suite 7200, Sacramento, CA 95817 (e-mail: [email protected]). Facial Plast Surg 2016;32:133–141. Abstract Congenital anomalies of the nose range from complete aplasia of the nose to duplications and nasal masses. Nasal development is the result of a complex embryo- logic patterning and fusion of multiple primordial structures. Loss of signaling proteins or failure of migration or proliferation can result in structural anomalies with significant Keywords cosmetic and functional consequences. Congenital anomalies of the nose can be ► nasal deformities categorized into four broad categories: (1) aplastic or hypoplastic, (2) hyperplastic or ► nasal dermoid duplications, (3) clefts, and (4) nasal masses. Our knowledge of the embryologic origin ► Tessier cleft of these anomalies helps dictate subsequent work-up for associated conditions, and the ► nasal cleft appropriate treatment or surgical approach to manage newborns and children with ► nasal hemangioma these anomalies. – Congenital anomalies of the nose are thought to be relatively side1 4 (►Fig. 1A, B). The medial processes will ultimately fuse, rare, affecting approximately 1 in every 20,000 to 40,000 live contributing to the nasal septum and the medial crura of the births.1 The exact incidence is difficult to quantify, as minor lower lateral cartilages.