Rheidae 1 Greater Rhea Rhea Americana Tinamous
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Birds of Reserva Ecológica Guapiaçu (REGUA)
Cotinga 33 The birds of Reserva Ecológica Guapiaçu (REGUA), Rio de Janeiro, Brazil Leonardo Pimentel and Fábio Olmos Received 30 September 2009; final revision accepted 15 December 2010 Cotinga 33 (2011): OL 8–24 published online 16 March 2011 É apresentada uma lista da avifauna da Reserva Ecológica de Guapiaçu (REGUA), uma reserva privada de 6.500 ha localizada no município de Cachoeiras de Macacu, vizinha ao Parque Estadual dos Três Picos, Estação Ecológica do Paraíso e Parque Nacional da Serra dos Órgãos, parte de um dos maiores conjuntos protegidos do Estado do Rio de Janeiro. Foram registradas um total de 450 espécies de aves, das quais 63 consideradas de interesse para conservação, como Leucopternis lacernulatus, Harpyhaliaetus coronatus, Triclaria malachitacea, Myrmotherula minor, Dacnis nigripes, Sporophila frontalis e S. falcirostris. A reserva também está desenvolvendo um projeto de reintrodução dos localmente extintos Crax blumembachii e Aburria jacutinga, e de reforço das populações locais de Tinamus solitarius. The Atlantic Forest of eastern Brazil and Some information has been published on neighbouring Argentina and Paraguay is among the birds of lower (90–500 m) elevations in the the most imperilled biomes in the world. At region10,13, but few areas have been subject to least 188 bird species are endemic to it, and 70 long-term surveys. Here we present the cumulative globally threatened birds occur there, most of them list of a privately protected area, Reserva Ecológica endemics4,8. The Atlantic Forest is not homogeneous Guapiaçu (REGUA), which includes both low-lying and both latitudinal and longitudinal gradients parts of the Serra dos Órgãos massif and nearby account for diverse associations of discrete habitats higher ground, now mostly incorporated within and associated bird communities. -
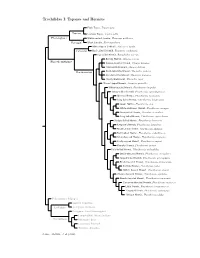
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

Nesting Behavior of Reddish Hermits (Phaethornis Ruber) and Occurrence of Wasp Cells in Nests
NESTING BEHAVIOR OF REDDISH HERMITS (PHAETHORNIS RUBER) AND OCCURRENCE OF WASP CELLS IN NESTS YOSHIKA ONIKI REDraSHHermits (Phaethornisruber) are small hummingbirdsof the forested tropical lowlands east of the Andes and south of the Orinoco (Meyer de Schauensee,1966: 161). Five birds mist-nettedat Belem (1 ø 28' S, 48ø 27' W, altitude 13 m) weighed2.0 to 2.5 g (average2.24 g). I studiedtheir nestingfrom 14 October1966 to October1967 at Belem, Brazil, in the Area de PesquisasEco16gicas do Guam•t (APEG) and MocamboForest reserves,in the Instituto de Pesquisase Experimentaqfio Agropecu•triasdo Norte (IPEAN). Names of forest types used and the Portugueseequivalents are: tidal swamp forest (vdrze'a), mature upland forest (terra-/irme) and secondgrowth (capoeira). In all casescapo.eira has been in mature upland situations. At Belem Phaethornisruber is commonall year in the lower levels of secondgrowth (capoeira) where thin branchesare plentiful. Isolated males call frequently from thin horizontal branches,never higher than 2.5-3.0 m. The male sits erect and wags his tail forward and backward as he squeaksa seriesof insectlike"pi-pi-pipipipipipi" notes, 18-20 times per minute; the first two or three notesare short and separated,the rest are run togetherrapidly. The bird sometimesstops calling for someseconds and flasheshis tongue in and out several times during the interval. I foundno singingassemblies of malehermits such as Davis (1934) describes for both the Reddishand Long-tailedHermits (Phaethornissuperciliosus). and Snow (1968) for the Little Hermit (P. longuemareus). Breeding season.--The monthly rainfall at Belem in the year of the study was 350 to 550 mm from January to May and 25 to 200 mm from June to December,with lows in October and November and highs in March and April. -

Birding the Atlantic Rainforest, South-East Brazil Itororo Lodge and Regua 11Th – 20Th March 2018
BIRDING THE ATLANTIC RAINFOREST, SOUTH-EAST BRAZIL ITORORO LODGE AND REGUA 11TH – 20TH MARCH 2018 White-barred Piculet (©Andy Foster) Guided and report compiled by Andy Foster www.serradostucanos.com.br Sunday 11th March The following 10 day tour was a private trip for a group of 4 friends. We all flew in from the UK on a BA flight landing the night of the 10th and stayed in the Linx Hotel located close to the International airport in Rio de Janeiro. We met up for breakfast at 07.00 and by 08.00 our driver had arrived to take us for the 2.5 hour drive to Itororo Lodge where we were to spend our first 6 nights birding the higher elevations of the Serra do Mar Mountains. On the journey up we saw Magnificent Frigatebird, Cocoi Heron, Great White Egret, Black-crowned Night Heron, Neotropic Cormorant and Roadside Hawk. By 10.30 we had arrived at the lodge and were greeted by Bettina and Rainer who would be our hosts for the next week. The feeders were busy at the lodge and we were soon picking up new species including Azure-shouldered Tanager, Brassy-breasted Tanager, Black-goggled Tanager, Sayaca Tanager, Ruby- crowned Tanager, Golden-chevroned Tanager, Magpie Tanager, Burnished-buff Tanager, Plain Parakeet, Maroon-bellied Parakeet, Rufous-bellied Thrush, Green-winged Saltator, Pale-breasted Thrush, Violet- capped Woodnymph, Black Jacobin, Scale-throated Hermit, Sombre Hummingbird, Brazilian Ruby and White-throated Hummingbird…. not bad for the first 30 minutes! We spent the last hour or so before lunch getting to grips with the feeder birds, we also picked up brief but good views of a Black-Hawk Eagle as it flew through the lodge gardens. -

Checklistccamp2016.Pdf
2 3 Participant’s Name: Tour Company: Date#1: / / Tour locations Date #2: / / Tour locations Date #3: / / Tour locations Date #4: / / Tour locations Date #5: / / Tour locations Date #6: / / Tour locations Date #7: / / Tour locations Date #8: / / Tour locations Codes used in Column A Codes Sample Species a = Abundant Red-lored Parrot c = Common White-headed Wren u = Uncommon Gray-cheeked Nunlet r = Rare Sapayoa vr = Very rare Wing-banded Antbird m = Migrant Bay-breasted Warbler x = Accidental Dwarf Cuckoo (E) = Endemic Stripe-cheeked Woodpecker Species marked with an asterisk (*) can be found in the birding areas visited on the tour outside of the immediate Canopy Camp property such as Nusagandi, San Francisco Reserve, El Real and Darien National Park/Cerro Pirre. Of course, 4with incredible biodiversity and changing environments, there is always the possibility to see species not listed here. If you have a sighting not on this list, please let us know! No. Bird Species 1A 2 3 4 5 6 7 8 Tinamous Great Tinamou u 1 Tinamus major Little Tinamou c 2 Crypturellus soui Ducks Black-bellied Whistling-Duck 3 Dendrocygna autumnalis u Muscovy Duck 4 Cairina moschata r Blue-winged Teal 5 Anas discors m Curassows, Guans & Chachalacas Gray-headed Chachalaca 6 Ortalis cinereiceps c Crested Guan 7 Penelope purpurascens u Great Curassow 8 Crax rubra r New World Quails Tawny-faced Quail 9 Rhynchortyx cinctus r* Marbled Wood-Quail 10 Odontophorus gujanensis r* Black-eared Wood-Quail 11 Odontophorus melanotis u Grebes Least Grebe 12 Tachybaptus dominicus u www.canopytower.com 3 BirdChecklist No. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

REGUA Bird List July 2020.Xlsx
Birds of REGUA/Aves da REGUA Updated July 2020. The taxonomy and nomenclature follows the Comitê Brasileiro de Registros Ornitológicos (CBRO), Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee, updated June 2015 - based on the checklist of the South American Classification Committee (SACC). Atualizado julho de 2020. A taxonomia e nomenclatura seguem o Comitê Brasileiro de Registros Ornitológicos (CBRO), Lista anotada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos, atualizada em junho de 2015 - fundamentada na lista do Comitê de Classificação da América do Sul (SACC). -

Houde2009chap64.Pdf
Cranes, rails, and allies (Gruiformes) Peter Houde of these features are subject to allometric scaling. Cranes Department of Biology, New Mexico State University, Box 30001 are exceptional migrators. While most rails are generally MSC 3AF, Las Cruces, NM 88003-8001, USA ([email protected]) more sedentary, they are nevertheless good dispersers. Many have secondarily evolved P ightlessness aJ er col- onizing remote oceanic islands. Other members of the Abstract Grues are nonmigratory. 7 ey include the A nfoots and The cranes, rails, and allies (Order Gruiformes) form a mor- sungrebe (Heliornithidae), with three species in as many phologically eclectic group of bird families typifi ed by poor genera that are distributed pantropically and disjunctly. species diversity and disjunct distributions. Molecular data Finfoots are foot-propelled swimmers of rivers and lakes. indicate that Gruiformes is not a natural group, but that it 7 eir toes, like those of coots, are lobate rather than pal- includes a evolutionary clade of six “core gruiform” fam- mate. Adzebills (Aptornithidae) include two recently ilies (Suborder Grues) and a separate pair of closely related extinct species of P ightless, turkey-sized, rail-like birds families (Suborder Eurypygae). The basal split of Grues into from New Zealand. Other extant Grues resemble small rail-like and crane-like lineages (Ralloidea and Gruoidea, cranes or are morphologically intermediate between respectively) occurred sometime near the Mesozoic– cranes and rails, and are exclusively neotropical. 7 ey Cenozoic boundary (66 million years ago, Ma), possibly on include three species in one genus of forest-dwelling the southern continents. Interfamilial diversifi cation within trumpeters (Psophiidae) and the monotypic Limpkin each of the ralloids, gruoids, and Eurypygae occurred within (Aramidae) of both forested and open wetlands. -

Table of Contents
AVIAN INVENTORY AND MONITORING REPORT LOMAS DE SIERPE ÁREA DE CONSERVACIÓN OSA PIEDRAS BLANCAS, OSA, PUNTARENAS, COSTA RICA PREPARED BY: KAREN M. LEAVELLE MSC. FOR: OSA CONSERVATION APRIL 2013 Baird’s Trogon TABLE OF CONTENTS INTRODUCTION 2 METHODS 2 STUDY AREA 2 BIRD SURVEYS 2 DISTANCE ESTIMATION 8 RESULTS 9 COMMUNITY COMPOSITION AND DENSITY 9 RESIDENT BIRD SPECIES 9 NEOTROPICAL MIGRATORY BIRD SPECIES 10 MELINA COMMUNITY COMPOSITION 14 FERN GROVE COMMUNITY COMPOSITION 15 MANAGEMENT RECOMMENDATIONS AND NEXT STEPS 16 LITERATURE CITED 18 TABLE 1: Species richness 9 TABLE 2: Cumulative list of Neotropical migratory bird species 2010-2013 11 TABLE 3: List of resident bird species 2013 11 TABLE 4: List of resident and Neotropical migratory bird species in the Melina plantation 2013 14 TABLE 5: List of resident and Neotropical migratory bird species in the Fern Grove plot 2013 16 TABLE 6: Densities 17 Appendix A: Cumulative list of resident and Neotropical migratory birds 2010-2013 19 RECOMMENDED CITATION Leavelle, K.M. 2013. Avian Inventory and Monitoring Report, Lomas de Sierpe, Área de Conservación Osa, Piedras Blancas, Costa Rica. Report prepared for Osa Conservation. p23. Washington, DC. 1 INTRODUCTION In concordance with the specific objective outlined for the development and continuance of scientific investigative activities on Osa Conservation’s Lomas de Sierpe property (Friends of the Osa and CATIE 2010), I conducted a formal avian inventory of resident and Neotropical migratory bird species from 9 March to 17 March 2013. Survey objectives were designed to assess avian community composition and estimate the density and abundance of individual target bird species of management and conservation concern in future survey years. -

Theristicus Caudatus;Argentina
The Condor96:99&1002 Q The cooper omith&gical society1994 BREEDING PERFORMANCE IN RELATION TO NEST-SITE SUBSTRATUM IN A BUFF-NECKED IBIS (THHWTICUS CA UDATUS) POPULATION IN PATAGONIA ’ Jose A. DONAZAR Estacidn Biologica de Doiiana, CSIC, Avda I%@Luisa s.n., 41013 Sevilla, Spain OLGA CEBALL~S Grupo de EstudiosBiologicos Ugarra, Carlos III 19, 31002 Pamplona, Spain ALEJANDRO TRAVAINI AND ALFJANDRO RODRIGUEZ EstacirinBiologica de Doiiana, CSIC Avda M Luisa s.n., 41013 Sevilla, Spain MARTIN FUNES Centro de Ecologia Aplicada de1Neuquen, Casilla de Correos92, 8371 Junin de 10sAndes, Neuquen, Argentina FERNANDO HIRAL~ Estacion Biologicade Dofiana, CSIC Avda M Luisa sn., 41013 Sevilla, Spain Abstract. In northern Argentinean Patagonia, Buff-necked Ibis (Theristicuscaudatus) nest on different substrata:cliffs, trees, and marsh vegetation. According to the ideal-free distribution hypothesis,this polymorphism may be due to the occupationofthe bestbreeding habitats by dominant individuals and the relegation of the subdominant birds to marginal substratawith a lower probability of achieving successfulbreeding. We investigatedwhether there were any variations in the breeding performance among colonies and whether these variations were related to the breeding substratum.Laying date varied from the third week of September to the last week of October, laying occurring earlier in colonies at lower elevations. Clutch size per colony varied between 1.8 and 2.0 (X 1.9, n = 106), but significant differences were not detected among colonies. Brood size per colony varied significantly, rangingbetween 1.3 and 2.0 (52= 1.8, n = 164). The substratumof breedingdid not influence variations in any of these three parameters. The physical condition of the chicks did not vary among substrata,but there was inter-colony variation in broods of two chicks. -

The Behavior and Ecology of Hermit Hummingbirds in the Kanaku Mountains, Guyana
THE BEHAVIOR AND ECOLOGY OF HERMIT HUMMINGBIRDS IN THE KANAKU MOUNTAINS, GUYANA. BARBARA K. SNOW OR nearly three months, 17 January to 5 April 1970, my husband and I F camped at the foot of the Kanaku Mountains in southern Guyana. Our camp was situated just inside the forest beside Karusu Creek, a tributary of Moco Moco Creek, at approximately 80 m above sea level. The period of our visit was the end of the main dry season which in this part of Guyana lasts approximately from September or October to April or May. Although we were both mainly occupied with other observations we hoped to accumulate as much information as possible on the hermit hummingbirds of the area, particularly their feeding niches, nesting and social organization. Previously, while living in Trinidad, we had studied various aspects of the behavior and biology of the three hermit hummingbirds resident there: the breeding season (D. W. Snow and B. K. Snow, 1964)) the behavior at singing assemblies of the Little Hermit (Phaethornis Zonguemareus) (D. W. Snow, 1968)) the feeding niches (B. K. Snow and D. W. Snow, 1972)) the social organization of the Hairy Hermit (Glaucis hirsuta) (B. K. Snow, 1973) and its breeding biology (D. W. Snow and B. K. Snow, 1973)) and the be- havior and breeding of the Guys’ Hermit (Phuethornis guy) (B. K. Snow, in press). A total of six hermit hummingbirds were seen in the Karusu Creek study area. Two species, Phuethornis uugusti and Phaethornis longuemureus, were extremely scarce. P. uugusti was seen feeding once, and what was presumably the same individual was trapped shortly afterwards. -

Baker2009chap58.Pdf
Ratites and tinamous (Paleognathae) Allan J. Baker a,b,* and Sérgio L. Pereiraa the ratites (5). Here, we review the phylogenetic relation- aDepartment of Natural Histor y, Royal Ontario Museum, 100 Queen’s ships and divergence times of the extant clades of ratites, Park Crescent, Toronto, ON, Canada; bDepartment of Ecology and the extinct moas and the tinamous. Evolutionary Biology, University of Toronto, Toronto, ON, Canada Longstanding debates about whether the paleognaths *To whom correspondence should be addressed (allanb@rom. are monophyletic or polyphyletic were not settled until on.ca) phylogenetic analyses were conducted on morphological characters (6–9), transferrins (10), chromosomes (11, 12), Abstract α-crystallin A sequences (13, 14), DNA–DNA hybrid- ization data (15, 16), and DNA sequences (e.g., 17–21). The Paleognathae is a monophyletic clade containing ~32 However, relationships among paleognaths are still not species and 12 genera of ratites and 46 species and nine resolved, with a recent morphological tree based on 2954 genera of tinamous. With the exception of nuclear genes, characters placing kiwis (Apterygidae) as the closest rela- there is strong molecular and morphological support for tives of the rest of the ratites (9), in agreement with other the close relationship of ratites and tinamous. Molecular morphological studies using smaller data sets ( 6–8, 22). time estimates with multiple fossil calibrations indicate that DNA sequence trees place kiwis in a derived clade with all six families originated in the Cretaceous (146–66 million the Emus and Cassowaries (Casuariiformes) (19–21, 23, years ago, Ma). The radiation of modern genera and species 24).