Colour and Textile Chemistry—A Lucky Career Choice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
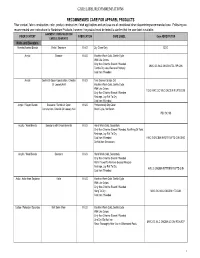
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

Review Article
Indian Journalof Fibre & Textile Research Vol. 29, December 2004, pp. 483-492 Review Article Development and processing of lyocell R B Chavan Department of Textile Technology, Indian Institute of Technology, New Delhi 110 016, India and A K Patra' The Technological Institute of Textile & Sciences, Bhiwani 127 02 1, India Received 20 June 2003; revised received and accepted December 2003 5 An account of Iyocell, covering the hindsight of its development and available brands has been reported. This wonder fibre surpasses all other cellulosic fibres in terms of properties, aesthetics and. quite importantly. ecology in manufacturing. Among the various names with which Iyocell is available. Tencel and Tencel A 100 are the prominent and widely used. Be sides dealing with the various attributes of Iyocell, the options for wet treatment of the fibre with reference to steps of proc essing. suitability of dyes and process parameters have also been addressed. Keywords: Fibrillation, Lyocell, Peach-skin effect. Tencel. Tencei A 100 IPC Code: CI. DO 1 F 2/00. D2 1 H 13/08 I nl. 7 1 Introduction solved solids. The viscose rayon manufacturing proc ' There has been a growing demand for absorbent fi ess is also energy-intensive. Besides this, viscose bres with the need hinging on comfort and fashion. rayon production has high labour demand, mainly due Since cotton production can not go beyond a particu to the complexity and number of steps involved in lar level due to limited land availability, the other ob converting pulp into rayon fibre. vious options are viscose and the likes. But again, Among the modified viscose fibres, high wet with the increasing awareness of ecofriendly con modulus (HWM) rayon involves relatively simple and cepts, viscose is not quite highly rated because its economical manufacturing process, but the zinc used manufacturing plants have inherent problem of efflu in this process is a known pollutant. -

A Comparison of the Patterns Formed by Primary Textile Structures and Their Photographic Abstraction
Rochester Institute of Technology RIT Scholar Works Theses 9-22-1976 A comparison of the patterns formed by primary textile structures and their photographic abstraction Pamela Perlman Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Perlman, Pamela, "A comparison of the patterns formed by primary textile structures and their photographic abstraction" (1976). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. Thesis Proposal for the Master of Fine Arts De gree Collee;e of Fine and Applj_ed .Arts Rochester Institute of Technology Title: A Comparison of the Fatterns Formed by Primary Textile structures and their Phot ographic Abstraction Submitted by: Pamela Anne Perlman Date: September 22, 1976 Thesis Co mm it te~: Nr . Donald Du jnowski I-Ir. I,l az Lenderman hr. Ed 1iiller Depart~ental Approval : Date :-:--g---li6~-r-71-b-r-/ ----- ---------~~~~~'~~~r------------------------- Chairman of the School for American Craftsme:l: ___-r-----,,~---- ____ Da t e : ---.:...,'?7~JtJ--J7~i,-=-~ ___ _ Chairr.ian of the Gr3.duate Prog:rarn: ------------------------~/~~/~. --- Date: ___________________~ /~~,~~;j~~, (~/_' ~i~/~: 7 / Final Committee Decision: Date: ----------------------- Thesis Proposal for the Master of Fine Arts Degree College of Fine and Applied Arts Rochester Institute of Technology Title: A Comparison of the Patterns Frmed by Primary Textile Structures and their Photographic Abstraction My concern in textiles is with structure and materials. I v/ould like to do v/all hangings based on primary textile structures such as knotting, looping, pile, balanced weaves, and tapestry. -

Uncompromising Protection. Unparalleled Comfort
UNCOMPROMISING PROTECTION. UNPARALLELED COMFORT. Discover Our North American Flash Fire Product Portfolio. 1 Our Commitment to FR Worker Safety At Westex by Milliken, worker safety is our priority. We’re leaders in secondary arc rated (AR) and flame resistant (FR) protection, backed by 150 years of Milliken innovation—and we go further than anyone else to ensure workers are protected, comfortable and able to return home safely each night. At the heart of our commitment is engineering: scientific expertise and advanced, custom-made equipment that guarantees flame resistance for the life of the garment. Market proven, with tens of millions of yards out in the field, our fabrics don’t just meet standards—they integrate safety and comfort in ways that were once considered impossible. But we don’t stop there with engineering and innovation. Through extensive educational outreach, we’ve helped millions of workers better understand arc flash, flash fire and other thermal hazards. Because helping workers feel confident in any and every condition comes down to innovation, exceptional engineering and education. For more information, visit westex.com/fabrics Details on Page 7 2 Westex protection delivers on the flash fire standard. The Need The Standard In oil, gas, chemical and petrochemical industries, the The National Fire Protection Association (NFPA) developed threat of flash fire exposures has necessitated the use NFPA® 2112 as the industry standard for flame-resistant of flame resistant clothing. Flame resistant clothing will garments designed to protect against Flash Fire. The official minimize burn injury and provide the worker a few seconds title for NFPA® 2112 was recently updated to “Standard on of escape time. -

Speciality Fibres
Speciality Fibres wool - global outlook what makes safil tick? nature inspires innovation in fabric renaissance for speciality fibre china rediscovers south african mohair who supplies the supplier? yarn & top dyeing sustainable wool production new normal in the year of the sheep BUYERS GUIDE TO WOOL 2015-2016 Welcome to Wool2Yarn Global - we have given our publication a new name! This new name reflects the growing number of yarn manufactures that are now an important facet of this publication. The new name also better reflects our expanding global readership with a wide profile from Acknowledgements & Thanks: wool grower to fabric, carpet and garment manufacturers in over 60 Alpha Tops Italy countries. American Sheep Association Australian Wool Testing Authority Our first publication was published in Russian in1986 when the Soviet British Wool Marketing Board Union was the biggest buyer of wool. After the collapse of the Soviet Campaign for Wool Canadian Wool Co-Operative Union this publication was superseded by a New Zealand / Australian Cape Wools South Africa English language edition that soon expanded to include profiles on China Wool Textile Association exporters in Peru, Uruguay, South Africa, Russia, UK and most of Federacion Lanera Argentina International Wool Textile Organisation Western Europe. Interwoollabs Mohair South Africa In 1999 we further expanded our publication list to include WOOL Nanjing Wool Market EXPORTER CHINA (now Wool2Yarn China) to reflect the growing New Zealand Wool Testing Authority importance of Asia and in particular China. This Chinese language SGS Wool Testing Authority magazine is a communication link between the global wool industry Uruguayan Wool Secretariat Wool Testing Authority Europe and the wool industry in China. -

Natural Fibers and Fiber-Based Materials in Biorefineries
Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 This report was issued on behalf of IEA Bioenergy Task 42. It provides an overview of various fiber sources, their properties and their relevance in biorefineries. Their status in the scientific literature and market aspects are discussed. The report provides information for a broader audience about opportunities to sustainably add value to biorefineries by considerin g fiber applications as possible alternatives to other usage paths. IEA Bioenergy Task 42: December 2018 Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 Report prepared by Julia Wenger, Tobias Stern, Josef-Peter Schöggl (University of Graz), René van Ree (Wageningen Food and Bio-based Research), Ugo De Corato, Isabella De Bari (ENEA), Geoff Bell (Microbiogen Australia Pty Ltd.), Heinz Stichnothe (Thünen Institute) With input from Jan van Dam, Martien van den Oever (Wageningen Food and Bio-based Research), Julia Graf (University of Graz), Henning Jørgensen (University of Copenhagen), Karin Fackler (Lenzing AG), Nicoletta Ravasio (CNR-ISTM), Michael Mandl (tbw research GesmbH), Borislava Kostova (formerly: U.S. Department of Energy) and many NTLs of IEA Bioenergy Task 42 in various discussions Disclaimer Whilst the information in this publication is derived from reliable sources, and reasonable care has been taken in its compilation, IEA Bioenergy, its Task42 Biorefinery and the authors of the publication cannot make any representation of warranty, expressed or implied, regarding the verity, accuracy, adequacy, or completeness of the information contained herein. IEA Bioenergy, its Task42 Biorefinery and the authors do not accept any liability towards the readers and users of the publication for any inaccuracy, error, or omission, regardless of the cause, or any damages resulting therefrom. -

Tub Dyeing Basics Step by Step Instructions on the Next Page G with Fiber Reactive Dyes
tub dyeing basics Step by step instructions on the next page g with Fiber Reactive Dyes Use this method to dye fabric or clothing, made of natural fibers one uniform or solid color. Also called Garment Dyeing or Vat Dyeing, this method can also be done in a washing machine. Fiber Reactive Dye is the dye of choice for all cellulose (plant) fibers, like cotton, Rayon, hemp, linen, Tencel®, Modal®, bamboo, etc. (For dyeing silk, wool and other protein fibers, see Dyeing Wool and Silk with Fiber Reactive Dyes on our website) The chemical bond of these dyes is permanent, so once all the excess dye is washed out an infant can chew on the fabric and it will not come off! Fiber Reactive Dyes work in lukewarm water so these directions can also be used to dye batik (waxed) fabrics in successive colors without fear of melting the wax. WHAT You’ll need: SALT & SODA ASH REQUIREMENTS • Fiber Reactive Dye • A bucket large enough The amount of Non-Iodized Salt and Soda Ash are for your item to move a function of the amount of water used. For each • Soda Ash around in, or a top pound of dry fabric you will need about 3 gallons of • Non-Iodized Salt loading washing machine warm water. The water must cover the fabric with • Urea (optional) • Pitcher & cup enough room for thorough, tangle-free stirring; oth- erwise you get uneven dyeing and streaks. For each • Calsolene Oil (optional) • Measuring cups 1½ gallons of water use 1½ cups of Non-Iodized • Synthrapol • Spoons Salt and 1/6 cup of Soda Ash. -

Using PRO MX Reactive Dye Please Read Directions Carefully Before Starting
BATIK using PRO MX Reactive Dye Please read directions carefully before starting. Batik is an ancient form of resist dyeing. Unwaxed areas of the fabric absorb dye while the waxed areas resist the dye, preserving the original color of the fabric. More wax is added to dyed areas preserving the new color and the fabric is dipped in another color of dye. Repeat this process until the design is completed then remove the wax. Designs may involve only one or many colors depending upon the number of times the hot wax is applied and the cloth is dipped into different colored dye baths. For additional information visit our web site at www.prochemicalanddye.com. Always use proper ventilation in your work area. Create a local exhaust system by putting a portable exhaust fan in a window, so it pulls air from the room to the outside. Heated wax releases irritating chemicals including acrolein and aldehydes. There is no approved MSHA/NIOSH filter for acrolein. A respirator is not a substitute for good ventilation. Heat wax to the lowest temperature at which it remains liquid. Do not leave hot wax unattended, as it is a fire hazard. Keep water away from the wax pot, as it will splatter. Always make sure you have a fire extinguisher or bucket of sand nearby in case of fire. Wax forms potentially hazardous vapors at high temperatures and may ignite. Do not use open flames, such as a gas or propane burner, instead use a crock pot or electric fry pan with temperature control. Make sure your hair is tied back and sleeves are rolled up when using heating equipment. -

Sustaining PICA for Future NASA Robotic Science Missions Including NF-4 and Discovery
Sustaining PICA for future NASA Robotic Science Missions including NF-4 and Discovery Mairead Stackpoole Ethiraj Venkatapathy Steven Violette NASA Ames Research Center NASA Ames Research Center Fiber Materials Inc. Moffett Field, CA 94035 Moffett Field, CA 94035 Biddeford, ME 04005 650-604-6199 650-604-4282 207-282-7062 Margaret.m.stackpoole.nasa.gov ethiraj.venkatapathy- [email protected] [email protected] Abstract—Phenolic Impregnated Carbon Ablator (PICA), was protected from the heat by a single piece net cast PICA invented in the mid 1990’s, is a low-density ablative thermal heatshield. OSIRIS REx, the first US mission to bring back protection material proven capable of meeting sample return samples from an asteroid (Bennu) to Earth, also uses a single mission needs from the moon, asteroids, comets and other piece PICA heatshield very similar to the Stardust design. In unrestricted class V destinations as well as for Mars. Its low addition to Stardust and OSIRIS-REx, PICA was used as the density and efficient performance characteristics have proven effective for use from Discovery to Flag-ship class missions. It is heatshield on the Mars Science Lab (MSL) in a tiled important that NASA maintain this thermal protection material configuration. The Mars 2020 mission heatshield design will capability and ensure its availability for future NASA use. The follow that of MSL and again use PICA in a tiled rayon based carbon precursor raw material used in PICA arrangement. A variant of PICA known as PICA-X is used preform manufacturing has experienced multiple supply chain as the heatshield TPS on the Dragon capsule. -

Responsible Production in the Lenzing Group
www.lenzing.com Responsibleproduction Lenzing Group Focus Responsible Production Responsible Production 2019Content Overview 4 Biorefinery – pulp production in the Lenzing Group 5 Biorefinery plant in Lenzing, Austria 6 Biorefinery plant in Paskov, Czech Republic 6 Pulp bleaching in the Lenzing Group 7 Overview of fiber technologies 8 Two-stage production process 9 EU Ecolabel 10 Lenzing’s viscose and modal production process 11 Lenzing’s responsible viscose criteria 12 Closing the loops in the viscose process: best practice 14 Lenzing’s lyocell production process 15 Further technologies 17 REFIBRA™ technology 17 Eco Filament technology 18 LENZING™ Web Technology 18 Fiber types 19 Shortcut fibers 19 Trilobal fibers 19 Incorporation of additives into the spinning mass 19 Definitions/glossary 20 References & suggestions for further reading 22 Focus Responsible Production Lenzing Group 2 Responsibleproduction This focus paper “Responsible Production” describes the biorefinery and fiber production processes. It provides an overview of the Lenzing Group’s manufacturing processes with particular regard to environmental aspects. Lenzing Group Focus Responsible Production Overview Forest Wood Pulp Fiber In recent years, interest in wood-based cellulosic fibers of over 90 percent, lower impurity levels, be bleached to a high- has increased due to their sustainability credentials. er level of brightness and have a more uniform molecular weight When the source of raw material is from sustainable for- distribution. estry, as proven by forest certificates, and state-of-the-art production processes are applied, wood-based cellulosic The Lenzing Group produces more than half of the pulp it requires fibers can have a very favorable environmental footprint. at its sites in Lenzing (Austria) and Paskov (Czech Republic). -

Comparative Study of Fastness Properties and Color Absorbance Criteria of Conventional and Avitera Reactive Dyeing on Cotton Knit Fabric
European Scientific Journal May 2016 edition vol.12, No.15 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 Comparative Study Of Fastness Properties And Color Absorbance Criteria Of Conventional And Avitera Reactive Dyeing On Cotton Knit Fabric Md. Abu Sufian B.Sc. in Textile Engineering,University of Chittagong, Bangladesh Md.Abdul Hannan M.Sc. in Textile Technology, University of Boras, Sweden Md. Masud Rana Muhmmad Zanibul Huq M.Sc. in Textile Engineering, Bangladesh University of Textiles, Bangladesh doi: 10.19044/esj.2016.v12n15p352 URL:http://dx.doi.org/10.19044/esj.2016.v12n15p352 Abstract 160 GSM Single Jersey cotton knitted fabric was dyed with conventional Remazol reactive dye and latest Avitera reactive dye (Huntsman). Detailed comparison of the process parameters and fastness properties of these dyed fabrics were studied. Investigation exposed that Avitera delivered better dyeing performance including fastness to washing, perspiration, rubbing than conventionally dyed fabrics. Concerning process parameters Avitera dye required less soda, salt and no addition of other auxiliaries. Also this new Avitera reactive dye is more eco-friendly, cost effective and energy saving than conventional Remazol reactive dye. CMC DE and Da* color deviation were significantly higher between the dyed samples. Again K/S value of Avitera dyed sample was superior to that of Remazol dyed samples as because of enhanced dye uptake. Sequentially reflectance and relative indicators of the latest reactive dyed samples were also experimented. Keywords: Avitera reactive dye, Remazol reactive dye, Color Fastness, Energy saving, K/S value, CMC DE, Reflectance. Introduction: The best dyes to apply on cotton fabric and other cellulose fibers are reactive dyes.