Euphyes Dukesi Lindsey Dukes’ Skipper Butterfly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Self-Repair and Self-Cleaning of the Lepidopteran Proboscis
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Self-Repair and Self-Cleaning of the Lepidopteran Proboscis Suellen Floyd Pometto Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Pometto, Suellen Floyd, "Self-Repair and Self-Cleaning of the Lepidopteran Proboscis" (2019). All Dissertations. 2452. https://tigerprints.clemson.edu/all_dissertations/2452 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. SELF-REPAIR AND SELF-CLEANING OF THE LEPIDOPTERAN PROBOSCIS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ENTOMOLOGY by Suellen Floyd Pometto August 2019 Accepted by: Dr. Peter H. Adler, Major Advisor and Committee Co-Chair Dr. Eric Benson, Committee Co-Chair Dr. Richard Blob Dr. Patrick Gerard i ABSTRACT The proboscis of butterflies and moths is a key innovation contributing to the high diversity of the order Lepidoptera. In addition to taking nectar from angiosperm sources, many species take up fluids from overripe or sound fruit, plant sap, animal dung, and moist soil. The proboscis is assembled after eclosion of the adult from the pupa by linking together two elongate galeae to form one tube with a single food canal. How do lepidopterans maintain the integrity and function of the proboscis while foraging from various substrates? The research questions included whether lepidopteran species are capable of total self- repair, how widespread the capability of self-repair is within the order, and whether the repaired proboscis is functional. -

Element Occurrence Data Standard
ELEMENT OCCURRENCE DATA STANDARD February 6, 2002 NatureServe in cooperation with the Network of Natural Heritage Programs and Conservation Data Centers iii Table of Contents PREFACE.....................................................................................................................................currently under revision ACKNOWLEDGMENTS.....................................................................................................currently under revision ABSTRACT..................................................................................................................................currently under revision 1 INTRODUCTION.........................................................................................................currently under revision 2 EO DEFINITION .................................................................................................................................................10 2.1 Principal EOs........................................................................................................................................................10 2.2 Sub-EOs.................................................................................................................................................................12 2.3 Feature Labels.......................................................................................................................................................13 2.4 Location Use Class..............................................................................................................................................13 -

ANOTHER NEW EUPHYES from the SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) the Southern Atlantic and Gulf Coastal Plains Ar
Journal of the Lepidopteri'ts' Society .50(1 ), 1996, 46- 53 ANOTHER NEW EUPHYES FROM THE SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) JOHN A. SHUEY The Nature ConselVancy, Indiana Field Office, 1330 West 38th Street, Indianapolis, Indiana 46208, USA ABSTRACT. The taxon Euphyes dukesi calhouni Shuey, new subspecies endemic to FIOlida, is described. This subspecies is amply differentiated from Euphyes dukesi dukesi and the two taxa are allopatric. In northeaste rn F lorida and sout eastern G eorgia, whe re their known ranges closely approach one another, there is almost no evidence of inte r gradation. Euphyes dukesi calhouni is limited to swamp habitats that support large stands of the sedge hostplants, various Rhynchospora and Carex specie:; (Cype raceae). Additional key words: biogeography, we tlands, conservation. The southern Atlantic and Gulf Coastal Plains are rich regions for wetland butterflies, especially for genera such as Euphyes, Poanes, and Problema. For example , as currently known, eight named Euphyes spe cies or subspecies occur in the wetlands of these coastal plains. Four of these taxa are restricted to the coastal plain: Euphyes palatka palatka (Edwards), Euphyes palatka klotsi Mille r, Harvey and Miller, Euphyes berryi (Bell), and Euphyes bayensis Shuey. Just as interesting as their limited coastal distributions is the presence in these wetland skippers of well differentiated p eripheral populations, many of which have only recently bee n recognized and described. These peripheral populations are most probably the end result of allopatric diffe rentiation. For example, Euphyes palatka klotsi repre sents its spe cies on a few of the lower Florida Keys, separated from the nominate mainland subspecies by just tens of miles. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Washington Butterfly Association Common Butterflies of the Puget Sound Region and Their Food Plants
Washington Butterfly Association [email protected] Pine White ( Neophasia menapia ) w ww.naba.org/chapters/nabaws / Identification : White with black forewing patch, black veins below. Flight Period : late June – early October, peak in August. Common Butterflies of the Puget Sound Region Favorite Nectar Plants : Goldenrod, and Their Food Plants - By David Droppers Pearly Everlasting, Asters, Thistles. Larval Host Plants : Ponderosa Pine, Western Tiger Swallowtail ( Papilio Lodgepole Pine, Douglas-Fir, among other conifers. rutulus ). Identification : Large. Yellow with black tiger stripes. Cabbage White ( Pieris rapae) Underside with some blue. Flight Identification : White, black wing tips and Period : mid April – late September, spots. Males have one spot, females two peak in June. Favorite Nectar Plants : spots. Flight Period : early March – early Mock Orange, Milkweeds, Thistles, November, peaks in May, July and large showy flowers. Larval Host September. Favorite Nectar Plants: Plants : Native Willows, Quaking Many, especially garden flowers, such as Oregano and Lavender. Aspen and other poplars, Red Alder Larval Host Plants : Garden Brassicae, especially broccoli and cabbage. Anise Swallowtail ( Papilio zelicaon) Identification : Large. Mostly black, Cedar Hairstreak ( Mitoura grynea ) centrally yellow, with row of blue dots Identification : Small. Varying brown above, below buff brown on hindwing. Flight Period : late with violet tint, variable white postmedian line, small tails on March – late September, peaks in May, hindwings. Flight Period : late March – early August, peaks in July-August. Favorite Nectar Plants : May-June. Favorite Nectar Plants : Goldenrods, Yarrow, Many flowers, mostly large and showy. Dandelion, Clovers, Red Flowering Currant. Larval Host Plants : Garden Parsley Larval Host Plants : Western Red Cedar, Incense Cedar and Dill, Angelica, Cow Parsnip, many others. -
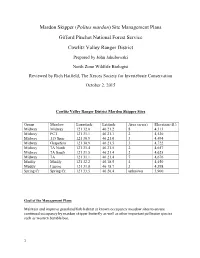
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Wet-Mesic Flatwoods Communitywet-Mesic Flatwoods, Abstract Page 1
Wet-mesic Flatwoods CommunityWet-mesic Flatwoods, Abstract Page 1 Historical Range Prevalent or likely prevalent Infrequent or likely infrequent Absent or likely absent Photo by Suzan L. Campbell Overview: Wet-mesic flatwoods is a somewhat Rank Justification: The acreage of wet-mesic poorly drained to poorly drained forest on mineral flatwoods present in Michigan circa 1800 is difficult soils dominated by a mixture of lowland and upland to determine because the community type has hardwoods. The community occurs exclusively on characteristics that overlap those of several of the glacial lakeplain in southeastern Lower Michigan, forest types mapped based on General Land Office where an impermeable clay layer in the soil profile (GLO) survey notes, primarily hardwood swamp and contributes to poor internal drainage. Seasonal beech-sugar maple forest (Comer et al. 1995a, Kost hydrologic fluctuations and windthrow are important et al. 2007). Analysis of GLO survey notes reveals natural disturbances that influence community structure, that lowland forest dominated by hardwoods covered species composition, and successional trajectory of wet- approximately 570,000 ha (1,400,000 ac) of southern mesic flatwoods. Lower Michigan circa 1800 (Comer et al. 1995a). These stands were characterized by mixed hardwoods Global and State Rank: G2G3/S2 (490,000 ha or 1,200,000 ac), black ash (77,000 ha or 190,000 ac), elm (5,300 ha or 13,000 ac), and silver Range: Flatwoods communities characterized by maple-red maple (4,000 ha or 10,000 ac). The majority relatively flat topography, slowly permeable to of lowland forest acreage in southern Lower Michigan impermeable subsurface soil layers, and seasonal was associated with stream and river floodplains, hydrologic fluctuation occur scattered throughout the and is classified as floodplain forest (Tepley et al. -

Phylogeny and Biogeography of Euphyes Scudder (Hesperiidae)
JOURNAL OF THE LEPIDOPTERISTS' SOCIETY Volume 47 1993 Number 4 Journal of the Lepidopterists Society 47(4), 1993, 261-278 PHYLOGENY AND BIOGEOGRAPHY OF EUPHYES SCUDDER (HESPERIIDAE) JOHN A. SHUEyl Great Lakes Environmental Center, 739 Hastings, Traverse City, Michigan 49684 ABSTRACT. The 20 species of Euphyes were analyzed phylogenetically and were found to fall into four monophyletic species groups, each of which is defined by one or more apomorphic characters. The peneia group contains Euphyes peneia (Godman), E. eberti Mielke, E. leptosema (Mabille), E. fumata Mielke, E. singularis (Herrich-Schiiffer), and E. cornelius (Latreille). The subferruginea group contains E. subferruginea Mielke, E. antra Evans, and E. cherra Evans. The dion group contains E. dion (Edwards), E. dukesi (Lindsey), E. bayensis Shuey, E. pilatka (Edwards), E. berryi (Bell), and E. con spicua (Edwards). The vestris group contains E. vestris (Boisduval), E. chamuli Freeman, E. bimacula (Grote and Robinson), and E arpa (Boisduval and Leconte). Euphyes ampa Evans could not be placed confidently w .nin this framework. Geographic distribution of each speci~s group suggests that exchange between South America and North America took place at least twice. The two Caribbean Basin species (E. singularis, E. cornelius) share a common ancestor with E. peneia, a species found in Central and South America. This suggests a vicariant event involving Central America and the Greater Antilles. The dion and vestris groups show strong patterns of alJopatric differentiation, suggesting that the isolation and subsequent differentiation of peripheral populations has played an important role in the development of the extant species. Additional key words: evolution, cladistics, wetlands, vicariance biogeography, pop ulation differentiation. -

Two New Records for the Appalachian Grizzled Skipper (Pyrgus Wyandot)
Banisteria, Number 24, 2004 © 2004 by the Virginia Natural History Society Status of the Appalachian Grizzled Skipper (Pyrgus centaureae wyandot) in Virginia Anne C. Chazal, Steven M. Roble, Christopher S. Hobson, and Katharine L. Derge1 Virginia Department of Conservation and Recreation Division of Natural Heritage 217 Governor Street Richmond, Virginia 23219 ABSTRACT The Appalachian grizzled skipper (Pyrgus centaureae wyandot) was documented historically (primarily from shale barren habitats) in 11 counties in Virginia. Between 1992 and 2002, staff of the Virginia Department of Conservation and Recreation, Division of Natural Heritage, conducted 175 surveys for P. c. wyandot at 75 sites in 12 counties. The species was observed at only six sites during these surveys, representing two new county records. All observations since 1992 combined account for <80 individuals. Due to forest succession and threats from gypsy moth control measures, all recent sites for P. c. wyandot in Virginia may be degrading in overall habitat quality. Key words: Lepidoptera, Pyrgus centaureae wyandot, conservation, shale barrens, Virginia. INTRODUCTION wyandot) in Virginia. Parshall (2002) provides a comprehensive review of the nomenclature and The Appalachian grizzled skipper (Pyrgus taxonomy of P. c. wyandot. Most authors classify this centaureae wyandot) has a rather fragmented range, skipper as a subspecies of the Holarctic Pyrgus occurring in northern Michigan as well as portions of centaureae (e.g., Opler & Krizek, 1984; Iftner et al., Ohio, Pennsylvania, Maryland, West Virginia, and 1992; Shuey, 1994; Allen, 1997; Opler, 1998; Virginia; isolated historical records are known from Glassberg, 1999; Parshall, 2002), although some Kentucky, New York, New Jersey, North Carolina, and lepidopterists treat it as a full species (Shapiro, 1974; the District of Columbia (Opler, 1998; NatureServe, Schweitzer, 1989; Gochfeld & Burger, 1997). -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -

Colourful Butterfly Wings: Scale Stacks, Iridescence and Sexual Dichromatism of Pieridae Doekele G
158 entomologische berichten 67(5) 2007 Colourful butterfly wings: scale stacks, iridescence and sexual dichromatism of Pieridae Doekele G. Stavenga Hein L. Leertouwer KEY WORDS Coliadinae, Pierinae, scattering, pterins Entomologische Berichten 67 (5): 158-164 The colour of butterflies is determined by the optical properties of their wing scales. The main scale structures, ridges and crossribs, scatter incident light. The scales of pierid butterflies have usually numerous pigmented beads, which absorb light at short wavelengths and enhance light scattering at long wavelengths. Males of many species of the pierid subfamily Coliadinae have ultraviolet-iridescent wings, because the scale ridges are structured into a multilayer reflector. The iridescence is combined with a yellow or orange-brown colouration, causing the common name of the subfamily, the yellows or sulfurs. In the subfamily Pierinae, iridescent wing tips are encountered in the males of most species of the Colotis-group and some species of the tribe Anthocharidini. The wing tips contain pigments absorbing short-wavelength light, resulting in yellow, orange or red colours. Iridescent wings are not found among the Pierini. The different wing colours can be understood from combinations of wavelength-dependent scattering, absorption and iridescence, which are characteristic for the species and sex. Introduction often complex and as yet poorly understood optical phenomena The colour of a butterfly wing depends on the interaction of encountered in lycaenids and papilionids. The Pieridae have light with the material of the wing and its spatial structure. But- two main subfamilies: Coliadinae and Pierinae. Within Pierinae, terfly wings consist of a wing substrate, upon which stacks of the tribes Pierini and Anthocharidini are distinguished, together light-scattering scales are arranged. -

Flexural Stiffness Patterns of Butterfly Wings (Papilionoidea)
35:61–77,Journal of Research1996 (2000) on the Lepidoptera 35:61–77, 1996 (2000) 61 Flexural stiffness patterns of butterfly wings (Papilionoidea) Scott J. Steppan Committee on Evolutionary Biology, University of Chicago, Chicago, IL 60637, USA., E-mail: [email protected] Abstract. A flying insect generates aerodynamic forces through the ac- tive manipulation of the wing and the “passive” properties of deformability and wing shape. To investigate these “passive” properties, the flexural stiffness of dried forewings belonging to 10 butterfly species was compared to the butterflies’ gross morphological parameters to determine allom- etric relationships. The results show that flexural stiffness scales with wing 3.9 loading to nearly the fourth power (pw ) and is highly correlated with wing area cubed (S3.1). The generalized map of flexural stiffness along the wing span for Vanessa cardui has a reduction in stiffness near the distal tip and a large reduction near the base. The distal regions of the wings are stiffer against forces applied to the ventral side, while the basal region is much stiffer against forces applied dorsally. The null hypothesis of structural isom- etry as the explanation for flexural stiffness scaling is rejected. Instead, selection for a consistent dynamic wing geometry (angular deflection) in flight may be a major factor controlling general wing stiffness and deformability. Possible relationships to aerodynamic and flight habit fac- tors are discussed. This study proposes a new approach to addressing the mechanics of insect flight and these preliminary results need to be tested using fresh wings and more thorough sampling. KEY WORDS: biomechanics, butterfly wings, flight, allometry, flexural stiff- ness, aerodynamics INTRODUCTION A flying insect generates aerodynamic forces primarily through the ac- tive manipulation of wing movements and the “passive” morphological prop- erties of deformability and wing shape.