Phylogeny and Biogeography of Euphyes Scudder (Hesperiidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paynes Creek Historic State Park
Paynes Creek Historic State Park Approved Plan Unit Management Plan STATE OF FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Recreation and Parks December 16, 2016 TABLE OF CONTENTS INTRODUCTION ...................................................................................1 PURPOSE AND SIGNIFICANCE OF THE PARK ....................................... 1 Park Significance ................................................................................1 PURPOSE AND SCOPE OF THE PLAN..................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................... 8 Management Authority and Responsibility .............................................. 8 Park Management Goals ...................................................................... 8 Management Coordination ................................................................... 9 Public Participation ..............................................................................9 Other Designations .............................................................................9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ................................................................................. 11 RESOURCE DESCRIPTION AND ASSESSMENT..................................... 12 Natural Resources ............................................................................. 12 Topography .................................................................................. 12 Geology ...................................................................................... -

Surveys for Dun Skipper (Euphyes Vestris) in the Harrison Lake Area, British Columbia, July 2009
Surveys for Dun Skipper (Euphyes vestris) in the Harrison Lake Area, British Columbia, July 2009 Report Citation: Parkinson, L., S.A. Blanchette, J. Heron. 2009. Surveys for Dun Skipper (Euphyes vestris) in the Harrison Lake Area, British Columbia, July 2009. B.C. Ministry of Environment, Ecosystems Branch, Wildlife Science Section, Vancouver, B.C. 51 pp. Cover illustration: Euphyes vestris, taken 2007, lower Fraser Valley, photo by Denis Knopp. Photographs may be used without permission for non-monetary and educational purposes, with credit to this report and photographer as the source. The cover photograph is credited to Denis Knopp. Contact Information for report: Jennifer Heron, Invertebrate Specialist, B.C. Ministry of Environment, Ecosystems Branch, Wildlife Science Section, 316 – 2202 Main Mall, Vancouver, B.C., Canada, V6T 1Z1. Phone: 604-222-6759. Email: [email protected] Acknowledgements Fieldwork was conducted by Laura Parkinson and Sophie-Anne Blanchette, B.C. Conservation Corps Invertebrate Species at Risk Crew. Jennifer Heron (B.C. Ministry of Environment) provided maps, planning and guidance for this project. The B.C. Invertebrate Species at Risk Inventory project was administered by the British Columbia Conservation Foundation (Joanne Neilson). Funding was provided by the B.C. Ministry of Environment through the B.C. Conservation Corp program (Ben Finkelstein, Manager and Bianka Sawicz, Program Coordinator), the B.C. Ministry of Environment Wildlife Science Section (Alec Dale, Manager) and Conservation Framework Funding (James Quayle, Manager). Joanne Neilson (B.C. Conservation Foundation) was a tremendous support to this project. This project links with concurrent invertebrate stewardship projects funded by the federal Habitat Stewardship Program for species at risk. -

ANOTHER NEW EUPHYES from the SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) the Southern Atlantic and Gulf Coastal Plains Ar
Journal of the Lepidopteri'ts' Society .50(1 ), 1996, 46- 53 ANOTHER NEW EUPHYES FROM THE SOUTHERN UNITED STATES COASTAL PLAIN (HESPERIIDAE) JOHN A. SHUEY The Nature ConselVancy, Indiana Field Office, 1330 West 38th Street, Indianapolis, Indiana 46208, USA ABSTRACT. The taxon Euphyes dukesi calhouni Shuey, new subspecies endemic to FIOlida, is described. This subspecies is amply differentiated from Euphyes dukesi dukesi and the two taxa are allopatric. In northeaste rn F lorida and sout eastern G eorgia, whe re their known ranges closely approach one another, there is almost no evidence of inte r gradation. Euphyes dukesi calhouni is limited to swamp habitats that support large stands of the sedge hostplants, various Rhynchospora and Carex specie:; (Cype raceae). Additional key words: biogeography, we tlands, conservation. The southern Atlantic and Gulf Coastal Plains are rich regions for wetland butterflies, especially for genera such as Euphyes, Poanes, and Problema. For example , as currently known, eight named Euphyes spe cies or subspecies occur in the wetlands of these coastal plains. Four of these taxa are restricted to the coastal plain: Euphyes palatka palatka (Edwards), Euphyes palatka klotsi Mille r, Harvey and Miller, Euphyes berryi (Bell), and Euphyes bayensis Shuey. Just as interesting as their limited coastal distributions is the presence in these wetland skippers of well differentiated p eripheral populations, many of which have only recently bee n recognized and described. These peripheral populations are most probably the end result of allopatric diffe rentiation. For example, Euphyes palatka klotsi repre sents its spe cies on a few of the lower Florida Keys, separated from the nominate mainland subspecies by just tens of miles. -

Butterflies and Moths of Brevard County, Florida, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

Section 5 References
Section 5 References 5.0 REFERENCES Akçakaya, H. R. and J. L. Atwood. 1997. A habitat-based metapopulation model of the California Gnatcatcher. Conservation Biology 11:422-434. Akçakaya, H.R. 1998. RAMAS GIS: Linking landscape data with population viability analysis (version 3.0). Applied Biomathematics, Setaauket, New York. Anderson, D.W. and J.W. Hickey. 1970. Eggshell changes in certain North American birds. Ed. K. H. Voous. Proc. (XVth) Inter. Ornith. Congress, pp 514-540. E.J. Brill, Leiden, Netherlands. Anderson, D.W., J.R. Jehl, Jr., R.W. Risebrough, L.A. Woods, Jr., L.R. Deweese, and W.G. Edgecomb. 1975. Brown pelicans: improved reproduction of the southern California coast. Science 190:806-808. Atwood, J.L. 1980. The United States distribution of the California black-tailed gnatcatcher. Western Birds 11: 65-78. Atwood, J.L. 1990. Status review of the California gnatcatcher (Polioptila californica). Unpublished Technical Report, Manomet Bird Observatory, Manomet, Massachusetts. Atwood, J.L. 1992. A maximum estimate of the California gnatcatcher’s population size in the United States. Western Birds. 23:1-9. Atwood, J.L. and J.S. Bolsinger. 1992. Elevational distribution of California gnatcatchers in the United States. Journal of Field Ornithology 63:159-168. Atwood, J.L., S.H. Tsai, C.H. Reynolds, M.R. Fugagli. 1998. Factors affecting estimates of California gnatcatcher territory size. Western Birds 29: 269-279. Baharav, D. 1975. Movement of the horned lizard Phrynosoma solare. Copeia 1975: 649-657. Barry, W.J. 1988. Management of sensitive plants in California state parks. Fremontia 16(2):16-20. Beauchamp, R.M. -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

The Butterfly Drawings by John Abbot in the Hargrett Rare Book and Manuscript Library, University of Georgia
VOLUME 61, NUMBER 3 125 Journal of the Lepidopterists’ Society 61(3), 2007, 125–137 THE BUTTERFLY DRAWINGS BY JOHN ABBOT IN THE HARGRETT RARE BOOK AND MANUSCRIPT LIBRARY, UNIVERSITY OF GEORGIA. JOHN V. C ALHOUN1 977 Wicks Dr., Palm Harbor, FL 34684 ABSTRACT. Artist-naturalist John Abbot completed 105 drawings of insects that are now deposited in the Hargrett Rare Book and Manu- script Library, University of Georgia. The provenance of these drawings is unknown, but available evidence dates them to ca. 1820–1825. The adults in the 32 butterfly drawings are identified and the figures of larvae and pupae are assessed for accuracy. The illustrated plants are also identified and their status as valid hosts is examined. Abbot’s accompanying notes are transcribed and analyzed. Erroneous figures of larvae, pupae, and hostplants are discussed using examples from the Hargrett Library. At least four of the butterfly species portrayed in the drawings were probably more widespread in eastern Georgia during Abbot’s lifetime. Additional key words: Larva, Lepidoptera, pupa, watercolors In 1776, the English artist-naturalist John Abbot METHODS (1751–ca.1840) arrived in Georgia, where he I visited the Hargrett Rare Book and Manuscript documented species of animals and plants for the next Library (University of Georgia) in April, 2005. Digital six decades. Living in Burke, Bullock, Chatham, and photographs were taken of John Abbot’s butterfly Screven Counties of eastern Georgia, he explored a drawings and their accompanying notes. The adult region roughly bound by the cities of Augusta and butterflies were identified and the figures compared Savannah, between the Oconee, Altamaha, and with those in other sets of Abbot’s drawings that are Savannah Rivers. -

Appendix a Sarah Tanedo/USFWS Common Tern with Chicks
Appendix A Sarah Tanedo/USFWS Common tern with chicks Animal Species Known or Suspected on Monomoy National Wildlife Refuge Table of Contents Table A.1. Fish Species Known or Suspected at Monomoy National Wildlife Refuge (NWR). ..........A-1 Table A.2. Reptile Species Known or Suspected on Monomoy NWR ............................A-8 Table A.3. Amphibian Species Known or Suspected on Monomoy NWR. ........................A-8 Table A.4. Bird Species Known or Suspected on Monomoy NWR. .............................A-9 Table A.5. Mammal Species Known or Suspected on Monomoy NWR. ......................... A-27 Table A.6. Butterfly and Moth Species Known or Suspected on Monomoy NWR. ................. A-30 Table A.7. Dragonfly and Damselfly Species Known or Suspected on Monomoy NWR. ............. A-31 Table A.8. Tiger Beetle Species Known or Suspected on Monomoy NWR. ...................... A-32 Table A.9. Crustacean Species Known or Suspected on Monomoy NWR. ...................... A-33 Table A.10. Bivalve Species Known or Suspected on Monomoy NWR. ......................... A-34 Table A.11. Miscellaneous Marine Invertebrate Species at Monomoy NWR. .................... A-35 Table A.12. Miscellaneous Terrestrial Invertebrates Known to be Present on Monomoy NWR. ........ A-37 Table A.13. Marine Worms Known or Suspected at Monomoy NWR. .......................... A-37 Literature Cited. ............................................................... A-40 Animal Species Known or Suspected on Monomoy National Wildlife Refuge Table A.1. Fish Species Known or Suspected at Monomoy National Wildlife Refuge (NWR). 5 15 4 1 1 2 3 6 6 Common Name Scientific Name Fall (%) (%) Rank Rank NOAA Spring Status Status Listing Federal Fisheries MA Legal Species MA Rarity AFS Status Occurrence Occurrence NALCC Rep. -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -
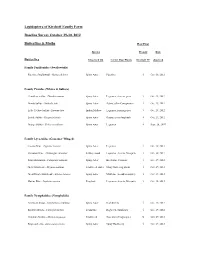
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Initial Environmental Review 2037, 2039 & 2043 Sea to Sky Highway (Hwy 99), Mount Currie, BC
Initial Environmental Review 2037, 2039 & 2043 Sea to Sky Highway (Hwy 99), Mount Currie, BC Prepared by: Cascade Environmental Resource Group Ltd. Unit 3 – 1005 Alpha Lake Road Whistler, BC V0N 1B1 Prepared for: Tom Laviolette Lil’wat Nation 321 No. 10 IR Road Mount Currie, BC Project Number: 467-06-02 Date: February 11, 2020 Acknowledgement In honour of the Lil’wat7ul, we acknowledge that we are within the unceded territory of the Lil’wat Nation. Statement of Limitations This Document was prepared by Cascade Environmental Resource Group Ltd. for Lil’wat Nation. Should this report contain an error or omission then the liability, if any, of Cascade Environmental Resource Group Ltd. should be limited to the fee received by Cascade Environmental Resource Group Ltd. for the preparation of this Document. Recommendations contained in this report reflect Cascade Environmental Resource Group Ltd.’s judgment in light of information available at the time of study. The accuracy of information provided to Cascade Environmental Resource Group Ltd. is not guaranteed. Neither all nor part of the contents of this report should be used by any party, other than the client, without the express written consent of Cascade Environmental Resource Group Ltd. This report was prepared for the client for the client’s own information and may not be used or relied upon by any other person unless that person is specifically named by Cascade Environmental Resource Group Ltd. as a beneficiary of the report, in which case the report may be used by the additional beneficiary Cascade Environmental Resource Group Ltd. -

Conservation of the Arogos Skipper, Atrytone Arogos Arogos (Lepidoptera: Hesperiidae) in Florida Marc C
Conservation of the Arogos Skipper, Atrytone arogos arogos (Lepidoptera: Hesperiidae) in Florida Marc C. Minno St. Johns River Water Management District P.O. Box 1429, Palatka, FL 32177 [email protected] Maria Minno Eco-Cognizant, Inc., 600 NW 35th Terrace, Gainesville, FL 32607 [email protected] ABSTRACT The Arogos skipper is a rare and declining butterfly found in native grassland habitats in the eastern and mid- western United States. Five distinct populations of the butterfly occur in specific parts of the range. Atrytone arogos arogos once occurred from southern South Carolina through eastern Georgia and peninsular Florida as far south as Miami. This butterfly is currently thought to be extirpated from South Carolina and Georgia. The six known sites in Florida for A. arogos arogos are public lands with dry prairie or longleaf pine savanna having an abundance of the larval host grass, Sorghastrum secundum. Colonies of the butterfly are threat- ened by catastrophic events such as wild fires, land management activities or no management, and the loss of genetic integrity. The dry prairie preserves of central Florida will be especially important to the recovery of the butterfly, since these are some of the largest and last remaining grasslands in the state. It may be possible to create new colonies of the Arogos skipper by releasing wild-caught females or captive-bred individuals into currently unoccupied areas of high quality habitat. INTRODUCTION tered colonies were found in New Jersey, North Carolina, South Carolina, Florida, and Mississippi. The three re- gions where the butterfly was most abundant included The Arogos skipper (Atrytone arogos) is a very locally the New Jersey pine barrens, peninsular Florida, and distributed butterfly that occurs only in the eastern and southeastern Mississippi.