X- and Γ-Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -

Glossary Derived From: Human Research Program Integrated Research Plan, Revision A, (January 2009)
Glossary derived from: Human Research Program Integrated Research Plan, Revision A, (January 2009). National Aeronautics and Space Administration, Johnson Space Center, Houston, Texas 77058, pages 232-280. Report No. 153: Information Needed to Make Radiation Protection Recommendations for Space Missions Beyond Low-Earth Orbit (2006). National Council on Radiation Protection and Measurements, pages 309-318. Reprinted with permission of the National Council on Radiation Protection and Measurements, http://NCRPonline.org . Managing Space Radiation Risk in the New Era of Space Exploration (2008). Committee on the Evaluation of Radiation Shielding for Space Exploration, National Research Council. National Academies Press, pages 111-118. -A- AAPM: American Association of Physicists in Medicine. absolute risk: Expression of excess risk due to exposure as the arithmetic difference between the risk among those exposed and that obtaining in the absence of exposure. absorbed dose (D): Average amount of energy imparted by ionizing particles to a unit mass of irradiated material in a volume sufficiently small to disregard variations in the radiation field but sufficiently large to average over statistical fluctuations in energy deposition, and where energy imparted is the difference between energy entering the volume and energy leaving the volume. The same dose has different consequences depending on the type of radiation delivered. Unit: gray (Gy), equivalent to 1 J/kg. ACE: Advanced Composition Explorer Mission, launched in 1997 and orbiting the L1 libration point to sample energetic particles arriving from the Sun and interstellar and galactic sources. It also provides continuous coverage of solar wind parameters and solar energetic particle intensities (space weather). When reporting space weather, it can provide an advance warning (about one hour) of geomagnetic storms that can overload power grids, disrupt communications on Earth, and present a hazard to astronauts. -

Cherenkov Radiation
TheThe CherenkovCherenkov effecteffect A charged particle traveling in a dielectric medium with n>1 radiates Cherenkov radiation B Wave front if its velocity is larger than the C phase velocity of light v>c/n or > 1/n (threshold) A β Charged particle The emission is due to an asymmetric polarization of the medium in front and at the rear of the particle, giving rise to a varying electric dipole momentum. dN Some of the particle energy is convertedγ = 491into light. A coherent wave front is dx generated moving at velocity v at an angle Θc If the media is transparent the Cherenkov light can be detected. If the particle is ultra-relativistic β~1 Θc = const and has max value c t AB n 1 cosθc = = = In water Θc = 43˚, in ice 41AC˚ βct βn 37 TheThe CherenkovCherenkov effecteffect The intensity of the Cherenkov radiation (number of photons per unit length of particle path and per unit of wave length) 2 2 2 2 2 Number of photons/L and radiation d N 4π z e 1 2πz 2 = 2 1 − 2 2 = 2 α sin ΘC Wavelength depends on charge dxdλ hcλ n β λ and velocity of particle 2πe2 α = Since the intensity is proportional to hc 1/λ2 short wavelengths dominate dN Using light detectors (photomultipliers)γ = sensitive491 in 400-700 nm for an ideally 100% efficient detector in the visibledx € 2 dNγ λ2 d Nγ 2 2 λ2 dλ 2 2 11 1 22 2 d 2 z sin 2 z sin 490393 zz sinsinΘc photons / cm = ∫ λ = π α ΘC ∫ 2 = π α ΘC 2 −− 2 = α ΘC λ1 λ1 dx dxdλ λ λλ1 λ2 d 2 N d 2 N dλ λ2 d 2 N = = dxdE dxdλ dE 2πhc dxdλ Energy loss is about 104 less hc 2πhc than 2 MeV/cm in water from € -
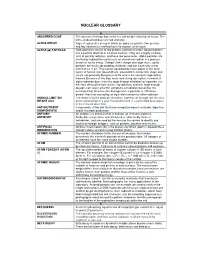
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Bone-Seeking Radiopharmaceuticals for Treatment of Osseous Metastases, Part 1: a Therapy with 223Ra-Dichloride
Downloaded from jnm.snmjournals.org by on June 26, 2020. For personal use only. CONTINUING EDUCATION Bone-Seeking Radiopharmaceuticals for Treatment of Osseous Metastases, Part 1: a Therapy with 223Ra-Dichloride Neeta Pandit-Taskar, Steven M. Larson, and Jorge A. Carrasquillo Molecular Imaging and Therapy Service, Memorial Sloan-Kettering Cancer Center, New York, New York Learning Objectives: On successful completion of this activity, participants should be able to (1) define the advantages and disadvantages of the use of a-emitting radionuclide 223Ra-dichloride in the treatment of painful metastatic osseous metastasis; (2) recognize the indications and contraindications and define the prerequisites for administration of 223Ra-dichloride; and (3) apply and integrate the treatment of osseous metastasis with 223Ra-dichloride in routine clinical practice. Financial Disclosure: Dr. Carrasquillo is a consultant/advisor for Bayer and Algeta. Dr. Larson is on the advisory board of ImaginAb, Molecular Imaging, and Perceptive Informatics; is a scientific advisor for Millennium and Progenics Pharmaceuticals, Inc., is a board member and the Chief Medical Officer of Claymore Technologies LLC; and has other ownership interest in Clinical Silica Technologies. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest. CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNMMI Web site (http:// www.snmmi.org/ce_online) through February 2017. -

Nuclear Fusion Enhances Cancer Cell Killing Efficacy in a Protontherapy Model
Nuclear fusion enhances cancer cell killing efficacy in a protontherapy model GAP Cirrone*, L Manti, D Margarone, L Giuffrida, A. Picciotto, G. Cuttone, G. Korn, V. Marchese, G. Milluzzo, G. Petringa, F. Perozziello, F. Romano, V. Scuderi * Corresponding author Abstract Protontherapy is hadrontherapy’s fastest-growing modality and a pillar in the battle against cancer. Hadrontherapy’s superiority lies in its inverted depth-dose profile, hence tumour-confined irradiation. Protons, however, lack distinct radiobiological advantages over photons or electrons. Higher LET (Linear Energy Transfer) 12C-ions can overcome cancer radioresistance: DNA lesion complexity increases with LET, resulting in efficient cell killing, i.e. higher Relative Biological Effectiveness (RBE). However, economic and radiobiological issues hamper 12C-ion clinical amenability. Thus, enhancing proton RBE is desirable. To this end, we exploited the p + 11Bà3a reaction to generate high-LET alpha particles with a clinical proton beam. To maximize the reaction rate, we used sodium borocaptate (BSH) with natural boron content. Boron-Neutron Capture Therapy (BNCT) uses 10B-enriched BSH for neutron irradiation-triggered alpha-particles. We recorded significantly increased cellular lethality and chromosome aberration complexity. A strategy combining protontherapy’s ballistic precision with the higher RBE promised by BNCT and 12C-ion therapy is thus demonstrated. 1 The urgent need for radical radiotherapy research to achieve improved tumour control in the context of reducing the risk of normal tissue toxicity and late-occurring sequelae, has driven the fast- growing development of cancer treatment by accelerated beams of charged particles (hadrontherapy) in recent decades (1). This appears to be particularly true for protontherapy, which has emerged as the most-rapidly expanding hadrontherapy approach, totalling over 100,000 patients treated thus far worldwide (2). -

Exposure Data
X-RADIATION AND γ-RADIATION 1. Exposure data 1.1 Occurrence 1.1.1 X-radiation X-rays are electromagnetic waves in the spectral range between the shortest ultraviolet (down to a few tens of electron volts) and γ-radiation (up to a few tens of mega electron volts) (see Figure 2, Overall introduction). The term γ-radiation is usually restricted to radiation originating from the atomic nucleus and from particle annihilation, while the term X-radiation covers photon emissions from electron shells. X-rays are emitted when charged particles are accelerated or decelerated, during transitions of electrons from the outer regions of the atomic shell to regions closer to the nucleus, and as bremsstrahlung, i.e. radiation produced when an electron collides with, or is deflected by, a positively charged nucleus. The resulting line spectra are characteristic for the corresponding element, whereas bremsstrahlung shows a conti- nuous spectrum with a steep border at the shortest wavelengths. Interaction of X-rays with matter is described by the Compton scattering and photoelectric effect and their resulting ionizing potentials, which lead to significant chemical and biological effects. Ions and radicals are produced in tissues from single photons and cause degradation and changes in covalent binding in macromolecules such as DNA. In other parts of the electromagnetic spectrum, below the spectra of ultraviolet and visible light, the single photon energies are too low to cause genotoxic × –μ⋅d effects. The intensity (I) of X-rays inside matter decreases according to I = I0 10 , where d is the depth and μ a coefficient specific to the interacting material and the corresponding wavelength. -

Office of Radiation Protection
How is Dose Measured? July 2002 Fact Sheet 320-058 Division of Environmental Health Office of Radiation Protection RADIATION DOSE When radioactive material decays and the transformation of the atom occurs there is characteristic energy that is released. This energy is released in the form of what we call radiation. There are different types of radiation, but they all serve the same general purpose, ridding the atom of excess energy after it transforms. These radiations travel until, by losing energy, they “stop”. Radiation loses its energy by interacting with atoms in its pathway and transferring energy to the atom during these interactions. When an interaction with radiation removes an electron from the atom it is called ionization. Other types of interactions include the excitation of an atom, the breaking of molecular bonds, and the heating of an atom or molecule. Ionization, excitation, and molecular bond breaking can cause biological damage; heat transfer does not necessarily cause biological damage. The purely physical event of energy deposited by a radiation in a given volume of material, i.e. tissue, is called the absorbed dose. The unit of absorbed dose is called the Rad, the international unit is the Gray (gy). The absorbed dose quantifies the amount of energy transferred to a volume of material, but it does not reflect the biological damage that potentially occurred. Because of the physics of radiation, the biological effect of the same amount of absorbed energy may vary according to the type of the radiation. A quality factor, Q was developed, to be able to compare absorbed doses from different radiation types. -

1. the Three Types of Nuclear Radiation Are Protons, Electrons, Neutrons
Page 1 of 8 This chapter has 53 questions. Scroll down to see and select individual questions or 0 questions at random and keep in order narrow the list using the checkboxes below. Multiple Choice Questions - (45) Topic: Nuclear reactions and nuclear fission - (4) Fill In The Blank Questions - (8) Topic: Nuclear reactors - (11) Odd Numbered - (27) Topic: Nuclear weapons and nuclear fusion - (9) Even Numbered - (26) Topic: Radioactive decay - (19) Accessibility: Keyboard Navigation - (45) Topic: The structure of the nucleus - (10) Difficulty: Easy - (46) Type: Conceptual - (50) Difficulty: Hard - (1) Type: Definition - (10) Difficulty: Medium - (6) Type: Numerical - (3) 1. The three types of nuclear radiation are protons, electrons, neutrons. electrical, strong nuclear, weak nuclear. radiation, convection, conduction. → gamma, beta, alpha. Accessibility: Keyboard Navigation Difficulty: Easy Topic: Radioactive decay Multiple Choice Question Type: Conceptual MC The three types of nuclear radiation are Type: Definition 2. The word "radioactive" means an atomic nucleus absorbs neutrons. there is significant interference between atomic radiation and radio reception. → there are nuclei present which will spontaneously emit nuclear radiation. an atom spontaneously captures an electron from a neighboring atom. Accessibility: Keyboard Navigation Difficulty: Easy Multiple Choice Question Topic: Radioactive decay MC The word radioactive means Type: Conceptual 3. The discovery of the neutron helped people understand → how two atoms of the same element can have different atomic masses. why the nucleus has a positive charge. how electrons are attracted to the nucleus. why the nucleus is much more massive than the electrons in an atom. Accessibility: Keyboard Navigation Difficulty: Easy Multiple Choice Question Topic: The structure of the nucleus MC The discovery of the neutron helped people u.. -

The Potential Detrimental Impact of Galactic Cosmic Radiation on Central Nervous System and Hematopoietic Stem Cells
THE POTENTIAL DETRIMENTAL IMPACT OF GALACTIC COSMIC RADIATION ON CENTRAL NERVOUS SYSTEM AND HEMATOPOIETIC STEM CELLS By RUTULKUMAR UPENDRABHAI PATEL Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Dr. Scott M. Welford, Ph.D Department of Pharmacology CASE WESTERN RESERVE UNIVERSITY January, 2019 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Rutulkumar Upendrabhai Patel Candidate for the Doctor of Philosophy degree *. (signed) Derek Taylor (Committee Chair) Scott M. Welford (Dissertation Advisor) Stanton L. Gerson (Committee Member) Marvin Nieman (Committee Member) Jennifer Yu (Committee Member) (date) December 3rd, 2018 *We also certify that written approval has been obtained for any proprietary material contained therein. ii Dedication I would like to dedicate this dissertation to my parents, Upendrabhai and Ujvalakumari Patel, who supported my wishes and ambitions despite being lived most of their lives in a lower-middle class family income. They sacrificed a lot to make sure a better life for their children. I would also like to dedicate this to my two sisters, Ekta and Vanita, for their support and encouragement over the years. iii Table of Contents Table of Contents ……………………………………………………………….. iv List of Figures ………………………………………………………………….. viii Acknowledgements ……………………………………………………………. xii Abstract ……………………………………………………………….…………. 1 Chapter 1: Introduction and Background ………………………………….. 3 1.1 Radiation, DNA Damage, and Carcinogenesis …………………………... 3 1.1.1 Space Radiation Environment and Induction of DNA Damage …………………………………………………………… 8 1.1.2 Radiation Induced Carcinogenesis ……………….................... 10 1.2 Hematopoietic Stem Cell Niche and Functions …………………………… 12 1.2.1 Low-LET Irradiation and HSC Injuries …………………………. 16 1.2.2 High-LET Irradiation Impact on HSCs …………………………. -

What Is Ionizing Radiation Fact Sheet
What is Ionizing Radiation? January 2003 Fact Sheet #3 Division of Environmental Health Office of Radiation Protection IONIZING RADIATION Ionizing radiation is radiation that has sufficient energy to remove orbital electrons from atoms, leading to the formation of ions. In this document, ionizing radiation will be referred to simply as radiation. One source of radiation is the nuclei of unstable atoms. For these radioactive atoms (also referred to as radionuclides or radioisotopes) to become more stable, the nuclei eject or emit subatomic particles and high-energy photons (gamma rays). This process is called radioactive decay. Unstable isotopes of radium, radon, uranium, and thorium, for example, exist naturally. Others are continually being made naturally or by human activities, such as the splitting of atoms in a nuclear reactor. Either way, they release ionizing radiation. Types of Ionizing Radiation ¨ Alpha Particle Radiation ¨ Beta Particle Radiation ¨ Gamma Ray Radiation ¨ X-Ray Radiation Alpha Particle Radiation An alpha particle consists of two neutrons and two protons ejected from the nucleus of an atom. The alpha particle is identical to the nucleus of a helium atom. Examples of alpha emitters are radium, radon, thorium, and uranium. Because alpha particles are charged and relatively heavy, they interact intensely with atoms in materials they encounter, giving up their energy over a very short range. In air, their travel distances are limited to approximately an inch. Alpha articles are easily shielded against and can be stopped by a single sheet of paper. Since alpha particles cannot penetrate the dead layer of the skin, they do not present a hazard from exposure external to the body. -

Radiation Properties Page 1 of 17
Radiation Properties Page 1 of 17 Radiation Properties The Atom The Bohr Model of the atom consists of a central nucleus composed of neutrons and protons surrounded by a number of orbital electrons equal to the number of protons. Protons are positively charged, while neutrons have no charge. Each has a mass of about 1 atomic mass unit or amu. Electrons are negatively charged and have mass of 0.00055 amu. The number of protons in a nucleus determines the element of the atom. For example, the number of protons in uranium is 92 while the number in neon is 10. The proton number is often referred to as Z. An element may have several isotopes. An isotope of an element is comprised of atoms containing the same number of protons as all other isotopes of that element, but each isotope has a different number of neutrons than other isotopes of that element. Isotopes may be expressed using the nomenclature Neon-20 or 2ONe10, where 20 represents the combined number of neutrons and protons in the atom (often referred to as the mass number A), and 10 represents the number of protons (the atomic number Z). While many isotopes are stable, others are not. Unstable isotopes normally release energy by undergoing nuclear transformations (also called decay) through one of several radioactive processes described later in this module. Elements are arranged in the periodic table with increasing Z. Radioisotopes are arranged by A and Z in the chart of the nuclides. Radiation Properties Page 2 of 17 Radiation Radiation is energy transmitted through space in the form of electromagnetic waves or energetic particles.