Introduction to Theranostics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -

Bone-Seeking Radiopharmaceuticals for Treatment of Osseous Metastases, Part 1: a Therapy with 223Ra-Dichloride
Downloaded from jnm.snmjournals.org by on June 26, 2020. For personal use only. CONTINUING EDUCATION Bone-Seeking Radiopharmaceuticals for Treatment of Osseous Metastases, Part 1: a Therapy with 223Ra-Dichloride Neeta Pandit-Taskar, Steven M. Larson, and Jorge A. Carrasquillo Molecular Imaging and Therapy Service, Memorial Sloan-Kettering Cancer Center, New York, New York Learning Objectives: On successful completion of this activity, participants should be able to (1) define the advantages and disadvantages of the use of a-emitting radionuclide 223Ra-dichloride in the treatment of painful metastatic osseous metastasis; (2) recognize the indications and contraindications and define the prerequisites for administration of 223Ra-dichloride; and (3) apply and integrate the treatment of osseous metastasis with 223Ra-dichloride in routine clinical practice. Financial Disclosure: Dr. Carrasquillo is a consultant/advisor for Bayer and Algeta. Dr. Larson is on the advisory board of ImaginAb, Molecular Imaging, and Perceptive Informatics; is a scientific advisor for Millennium and Progenics Pharmaceuticals, Inc., is a board member and the Chief Medical Officer of Claymore Technologies LLC; and has other ownership interest in Clinical Silica Technologies. The authors of this article have indicated no other relevant relationships that could be perceived as a real or apparent conflict of interest. CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNMMI Web site (http:// www.snmmi.org/ce_online) through February 2017. -

Appropriate Use Criteria for Bone Scintigraphy in Prostate and Breast Cancer
Appropriate Use Criteria for Bone Scintigraphy in Prostate and Breast Cancer Kevin J. Donohoe1, Erica J. Cohen1, Francesco Giammarile2, Erin Grady1, Bennett S. Greenspan1, Robert E. Henkin1, John Millstine1, Gary T. Smith1, Sandy Srinivas3, Julie Kauffman1, and Sukhjeet Ahuja1 1Society of Nuclear Medicine and Molecular Imaging; 2European Association of Nuclear Medicine; and 3American Society of Clinical Oncology EXECUTIVE SUMMARY INTRODUCTION Nuclear medicine imaging studies are essential to the di- The following document describes the appropriate use of agnosis and management of many diseases, including neoplastic nuclear medicine bone scintigraphy in patients with breast and disease (1). Modern imaging studies allow noninvasive exami- prostate cancer. The authors have tried to cover the most common nation of anatomic and physiologic processes that often change clinical scenarios for bone scintigraphy in patients with these two patient management and improve outcomes. The ready availabil- common malignancies. However, the reader is reminded that a ity and high sensitivity of medical imaging in conjunction with patient may present with variations of the scenarios covered here, concerns about missed diagnoses has, at times, resulted in in- or with signs or symptoms not described, for which bone appropriate use of the technology. This has resulted in an un- scintigraphy may still be indicated. This document is presented necessary financial burden on the health-care system and in some to assist health-care practitioners considering bone scintigraphy cases unnecessary exposure to ionizing radiation (1–5). Incon- for patients with breast and prostate cancer; however, each patient sistent use of specific imaging procedures for similar clinical is unique, as is each patient’s clinical presentation, and therefore scenarios has prompted a push for consensus documents outlin- this document cannot replace clinical judgement. -

PERPUSTAKAAN PRIBADI AN-NUR Targeted Radionuclide Tumor Therapy Torgny Stigbrand • Jörgen Carlsson Gregory P
PERPUSTAKAAN PRIBADI AN-NUR Targeted Radionuclide Tumor Therapy Torgny Stigbrand • Jörgen Carlsson Gregory P. Adams Editors Targeted Radionuclide Tumor Therapy Biological Aspects Editors Torgny Stigbrand Gregory P. Adams University of Umea Fox Chase Cancer Center Department of Immunology Department of Medical Oncology Umea, Sweden Philadelphia, USA Jörgen Carlsson Uppsala University Rudbeck Laboratory Uppsala, Sweden ISBN 978-1-4020-8695-3 e-ISBN 978-1-4020-8696-0 Library of Congress Control Number: 2008931003 © 2008 Springer Science + Business Media B.V. No part of this work may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording or otherwise, without written permission from the Publisher, with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Printed on acid-free paper 9 8 7 6 5 4 3 2 1 springer.com Preface The last three decades have provided opportunities to explore the potential of treating malignant diseases with antibodies or other targeting molecules labelled with nuclides. While considerable advances have been reported, there is still a signifi- cant amount of work left to accomplish before our ambitions can be achieved. It now seems timely to review the accomplishments achieved to date and to clarify the challenges that remain. The choice of radionuclide, the conjugation pro- cedure employed, and the selection of suitable targets were early issues that were faced by our field that still persist, however we can now tackle these obstacles with significantly better insight. -
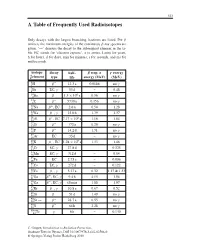
A Table of Frequently Used Radioisotopes
323 A Table of Frequently Used Radioisotopes Only decays with the largest branching fractions are listed. For β emitters the maximum energies of the continuous β-ray spectra are given. ‘→’ denotes the decay to the subsequent element in the ta- ble. EC stands for ‘electron capture’, a (= annus, Latin) for years, h for hours, d for days, min for minutes, s for seconds, and ms for milliseconds. isotope decay half- β resp. α γ energy A Z element type life energy (MeV) (MeV) 3 β− . γ 1H 12 3a 0.0186 no 7 γ 4Be EC, 53 d – 0.48 10 β− . × 6 γ 4Be 1 5 10 a 0.56 no 14 β− γ 6C 5730 a 0.156 no 22 β+ . 11Na ,EC 2 6a 0.54 1.28 24 β− γ . 11Na , 15 0h 1.39 1.37 26 β+ . × 5 13Al ,EC 7 17 10 a 1.16 1.84 32 β− γ 14Si 172 a 0.20 no 32 β− . γ 15P 14 2d 1.71 no 37 γ 18Ar EC 35 d – no 40 β− . × 9 19K ,EC 1 28 10 a 1.33 1.46 51 γ . 24Cr EC, 27 8d – 0.325 54 γ 25Mn EC, 312 d – 0.84 55 . 26Fe EC 2 73 a – 0.006 57 γ 27Co EC, 272 d – 0.122 60 β− γ . 27Co , 5 27 a 0.32 1.17 & 1.33 66 β+ γ . 31Ga , EC, 9 4h 4.15 1.04 68 β− γ 31Ga , EC, 68 min 1.88 1.07 85 β− γ . -

Radium-226, Releases Half of Its Radiation in About 1,600 Years
TOXICOLOGICAL PROFILE FOR RADIUM Agency for Toxic Substances and Disease Registry U.S. Public Health Service In collaboration with: U.S. Environmental Protection Agency December 1990 ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorbement by the Agency for Toxic Substances and Disease Registry. 1 1. PUBLIC HEALTH STATEMENT This Statement was prepared to give you information about radium and to emphasize the human health effects that may result from exposure to it. The Environmental Protection Agency (EPA) has identified 1,177 sites on its National Priorities List (NPL). Radium has been found above background levels at 18 of these sites. However, we do not know how many of the 1,177 NPL sites have been evaluated for radium. As EPA evaluates more sites, the number of sites at which radium is found above background levels may change. The information is important for you because radium may cause harmful health effects and because these sites are potential or actual sources of human exposure to radium. When a radioactive chemical is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment as a radioactive chemical emission. This emission, which is also called a release, does not always lead to exposure. You can be exposed to a radioactive chemical when you come into contact with that chemical alone or with a substance that contains it. You may be exposed to it in the environment by breathing, eating, or drinking substances containing the radioactive chemical or from skin contact with it. -
Bone-Targeted Imaging and Radionuclide Therapy in Prostate Cancer
Bone-Targeted Imaging and Radionuclide Therapy in Prostate Cancer Andrei H. Iagaru1, Erik Mittra1, Patrick M. Colletti2, and Hossein Jadvar2 1Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Stanford University, Stanford, California; and 2Division of Nuclear Medicine, Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, California have prompted much interest in the use of PET in this clinical Although selective metabolic and receptor-based molecular agents setting (2). will surely be included in the future of prostate cancer diagnosis and therapy, currently available inorganic compounds—such as 18F-NaF 18F-NAF PET IN PC 223 for the diagnosis of bony disease and RaCl2 for the therapy of bone 18 metastases—were recently shown to be superior to standard 99mTc- F-NaF is a positron-emitting radiopharmaceutical that was phosphonates for diagnosis and 153Sm-ethylenediaminetetramethylene used for skeletal scintigraphy in the 1970s. Clinical use was lim- 89 18 phosphonate or SrCl2 for therapy. The advantages of F-NaF in- ited then because of logistic difficulties in delivering tracers with a clude improved lesion detection and, when used in combination with half-life of 110 min and the absence of PET technology. 18F-NaF CT, improved diagnostic confidence and specificity. In addition to was replaced in the late 1970s by 99mTc-labeled phosphonates, α 223 being the first approved -emitter, RaCl2 is the first radiopharma- which had improved g-camera compatibilities (3,4). As an analog ceutical to show an increase in overall survival, a decrease in skeletal of the hydroxyl group found in hydroxyapatite bone crystals, 18F- events, palliation of bone pain, and a low profile of adverse reactions NaF is an avid bone seeker. -
Radiation Volume 100 D a Review of Human Carcinogens
RADIATION volume 100 D A review of humAn cArcinogens This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 2-9 June 2009 LYON, FRANCE - 2012 iArc monogrAphs on the evAluAtion of cArcinogenic risks to humAns X- AND γ-RADIATION X-and γ-radiation were considered by a previous IARC Working Group in 1999 (IARC, 2000). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.1 X- and γ-rays X- and γ-rays are both electromagnetic 1.1 Physical properties radiations distinguished mainly by their origin. X-rays are photons emitted from the electron Radiation sources can be either external to shells surrounding the atomic nucleus or during the body, such as medical X-rays, or through the slowing down of electrons or other charged deposition on the Earth’s surface, or internal. particles. The term γ-rays is usually applied to Internal exposure can result from the ingestion of radiation originating from the atomic nucleus, contaminated foods, inhalation, dermal absorp- and from particle annihilation. The energy tion, or injection of radionuclides. The effects of ranges of X- and γ-rays overlap considerably with radiation are directly related to the dose that an X-rays having energies upwards from a few tens organ receives, and any differences between the of eV (the shortest ultraviolet wavelengths), and effects of external and internal sources is in large γ-ray energies extending up to a few tens of MeV. -
Page “SPECT CT REVIEW COURSE” ( 75 Hrs. Category a Credits)
“SPECT CT REVIEW COURSE” ( 75 Hrs. Category A Credits) SYLLABUS Timothy K. Marshel, MBA, R.T. (R)(N)(CT)(MR)(NCT)(PET)(CNMT) The PET/CT Training Institute, Inc. Overview: This 75 hr online self-study Aschronous Learning Management System course will cover various topics of SPECT, CT, and SPECT/CT. SPECT/CT combines two complementary advanced imaging technologies- Single Photon Emission Computed Tomography (SPECT) and Computed Axial Tomography (CT) into a single gantry scanner that can acquire SPECT and CT data simultaneously. The simultaneous overlapping of acquisition of SPECT and CT data enables new opportunities for physicians who need diagnostic imaging of patients with cancer, brain disorders, and heart disease, among other diseases and neurological issues. This course will cover topics of SPECT/CT, ranging from technical developments to clinical applications. Technical developments will include understanding basic SPECT and CT approaches to attenuation correction and motion correction. This course will provide a broad overview of clinical applications of both SPECT and CT within the body and brain. The intended advantage of this course for Technologist is to allow only one technologist to perform both scanning procedures. Audience: The target audience for this online course is primarily Imaging Technologist. 1 | P a g e Contents Module I: Fundamentals of SPECT ........................................................................................................................3 Lecture 1: SPECT Terminology (90 minutes) .............................................................................................3 -

Bone-Seeking Targeted Radio-Nuclide Therapy (BT-RNT) In
dicine Me & r R a a d le i c a u t i o Journal of N n f T o l h Nair, et al., J Nucl Med Radiat Ther 2014, 6:1 a e n r r ISSN: 2155-9619a u p o y J Nuclear Medicine & Radiation Therapy DOI: 10.4172/2155-9619.1000202 Review Article Open Access Bone-Seeking Targeted Radio-Nuclide Therapy (BT-RNT) in Management of Metastatic Castration-Resistant Prostate Cancer (mCRPC): Shifting from Palliation to Improving Survival Vimoj J Nair1, Colin Malone2, Patricia Moretto3, Eugene Leung3 and Shawn Malone1* 1Division of Radiation Oncology, University of Ottawa and Ottawa Hospital, Ottawa, Canada 2Division of Medical Oncology, University of Ottawa and Ottawa Hospital, Ottawa, Canada 3Division of Nuclear Medicine, University of Ottawa and Ottawa Hospital, Ottawa, Canada *Corresponding author: Shawn Malone, Associate Professor, Department of Radiation Oncology, The Ottawa Hospital Cancer Centre, 501 Smyth Road, Ottawa, Ontario, K1H 8L6, Canada, Tel: 001-613-737-7700; E-mail: [email protected] Received date: Oct 16, 2014, Accepted date: Nov 28, 2014, Publication date: Dec 01, 2014 Copyright: © 2014 Nair VJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Background: The purpose of this article is to review the role of bone-seeking targeted radionuclide therapy (BT- RNT) in metastatic prostate cancer. The mechanisms of actions, radiobiology and clinical benefits of BT-RNTs will be reviewed. -

X- and Γ-Radiation
X- AND γ-RADIATION X-and γ-radiation were considered by a previous IARC Working Group in 1999 (IARC, 2000). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.1 X- and γ-rays X- and γ-rays are both electromagnetic 1.1 Physical properties radiations distinguished mainly by their origin. X-rays are photons emitted from the electron Radiation sources can be either external to shells surrounding the atomic nucleus or during the body, such as medical X-rays, or through the slowing down of electrons or other charged deposition on the Earth’s surface, or internal. particles. The term γ-rays is usually applied to Internal exposure can result from the ingestion of radiation originating from the atomic nucleus, contaminated foods, inhalation, dermal absorp- and from particle annihilation. The energy tion, or injection of radionuclides. The effects of ranges of X- and γ-rays overlap considerably with radiation are directly related to the dose that an X-rays having energies upwards from a few tens organ receives, and any differences between the of eV (the shortest ultraviolet wavelengths), and effects of external and internal sources is in large γ-ray energies extending up to a few tens of MeV. part related to the distribution of dose within and among body organs (IARC, 2001). (a) X-rays The activity of a radionuclide is defined as the Characteristic X-rays are emitted during number of nuclear transformations occurring per transitions of electrons in excited atomic shells unit of time. -

MIRD Pamphlet No. 22 - Radiobiology and Dosimetry of Alpha- Particle Emitters for Targeted Radionuclide Therapy
Alpha-Particle Emitter Dosimetry MIRD Pamphlet No. 22 - Radiobiology and Dosimetry of Alpha- Particle Emitters for Targeted Radionuclide Therapy George Sgouros1, John C. Roeske2, Michael R. McDevitt3, Stig Palm4, Barry J. Allen5, Darrell R. Fisher6, A. Bertrand Brill7, Hong Song1, Roger W. Howell8, Gamal Akabani9 1Radiology and Radiological Science, Johns Hopkins University, Baltimore MD 2Radiation Oncology, Loyola University Medical Center, Maywood IL 3Medicine and Radiology, Memorial Sloan-Kettering Cancer Center, New York NY 4International Atomic Energy Agency, Vienna, Austria 5Centre for Experimental Radiation Oncology, St. George Cancer Centre, Kagarah, Australia 6Radioisotopes Program, Pacific Northwest National Laboratory, Richland WA 7Department of Radiology, Vanderbilt University, Nashville TN 8Division of Radiation Research, Department of Radiology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark NJ 9Food and Drug Administration, Rockville MD In collaboration with the SNM MIRD Committee: Wesley E. Bolch, A Bertrand Brill, Darrell R. Fisher, Roger W. Howell, Ruby F. Meredith, George Sgouros (Chairman), Barry W. Wessels, Pat B. Zanzonico Correspondence and reprint requests to: George Sgouros, Ph.D. Department of Radiology and Radiological Science CRB II 4M61 / 1550 Orleans St Johns Hopkins University, School of Medicine Baltimore MD 21231 410 614 0116 (voice); 413 487-3753 (FAX) [email protected] (e-mail) - 1 - Alpha-Particle Emitter Dosimetry INDEX A B S T R A C T.........................................................................................................................