Viral Hepatitis an Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Viral Hepatitis Testing Effective Date: January 1, 2012
Viral Hepatitis Testing Effective Date: January 1, 2012 Scope This guideline provides guidance for the use of laboratory tests to diagnose acute and chronic viral hepatitis in adults (> 19 years) in the primary care setting. General Considerations for Ordering Laboratory Tests Prior to ordering tests for hepatitis, consider the patient’s history, age, risk factors (see below), hepatitis vaccination status, and any available previous hepatitis test results. Risk Factors for Viral Hepatitis include: • Substance use (includes sharing drug snorting, smoking or injection equipment) • High-risk sexual activity or sexual partner with viral hepatitis • Travel to or from high-risk hepatitis endemic areas or exposure during a local outbreak • Immigration from hepatitis B and/or C endemic countries • Household contact with an infected person especially if personal items (e.g., razors, toothbrushes, nail clippers) are shared • Recipient of unscreened blood products* • Needle-stick injury or other occupational exposure (e.g., healthcare workers) • Children born to mothers with chronic hepatitis B or C infection • Attendance at daycare • Contaminated food or water (hepatitis A only) • Tattoos and body piercing • History of incarceration • HIV or other sexually transmitted infection • Hemodialysis *screening of donated blood products for hepatitis C (anti-HCV) began in 1990 in Canada.1 Types of Viral Hepatitis Hepatitis A: causes acute but not chronic hepatitis Hepatitis B: causes acute and chronic hepatitis Hepatitis C: causes chronic hepatitis but rarely manifests as acute hepatitis Hepatitis D: rare and only occurs in patients infected with hepatitis B Hepatitis E: clinically similar to hepatitis A, mostly restricted to endemic areas and occasionally causes chronic infection in immunosuppressed people Others: e.g. -

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -
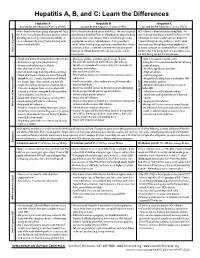
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days. -

HEPATITIS D the Most Virulent Hepatitis Virus of All
HEPATITIS D The Most Virulent Hepatitis Virus Of All The hepatitis D virus is perhaps the most unique of all hepatitis viruses, and also the most virulent. As a virus, it is flawed. The hepatitis D virus (HDV) cannot replicate and infect someone unless the person is already infected with the hepatitis B virus (HBV). The hepatitis D virus requires the outer coating of the hepatitis B virus—called the surface antigen—in order to reproduce itself in a human host. The virus currently infects 15 million worldwide, nearly all adults, and it is most common among injecting drug user populations and in countries bordering the Mediterranean Sea. Most children with HDV infection live in Italy and Greece, with a few in northern Africa. There are no reports or estimates of There are two types of HDV infection, the number of children infected with coinfection and super-infection: HDV worldwide. • Coinfection occurs when a patient is Despite widespread hepatitis B in simultaneously infected with HDV and Asian populations, HDV infection is HBV. The majority of these patients virtually unknown there and appears completely recover but there is a higher rate of fulminant hepatic failure and primarily in injecting drug user death than with HBV infection alone. populations. • Super-infection occurs when someone HDV is transmitted through blood and with an existing chronic HBV infection body fluids, similar to the hepatitis B becomes infected with HDV. These patients usually experience a sudden virus. Hepatitis D symptoms are worsening of liver disease. Patients with similar to other viral hepatitis diseases hepatitis B who become chronically and include jaundice, fever, malaise, infected with HDV experience a very dark urine and nausea. -

HEPATITIS D (Viral Hepatitis D, Hepatitis Delta Virus, Delta Agent Hepatitis, Delta Associated Hepatitis)
FACT SHEET HEPATITIS D (Viral hepatitis D, Hepatitis delta virus, Delta agent hepatitis, Delta associated hepatitis) What is hepatitis D? Hepatitis D is a virus that infects the liver. Hepatitis D, or Delta hepatitis, is always associated with a hepatitis B infection. A person may recover from Delta hepatitis or it may progress to chronic hepatitis. Who gets hepatitis D? Hepatitis D can only occur if the person has hepatitis B. Hepatitis D virus (HDV) and hepatitis B virus (HBV) may infect a person at the same time or HDV infection may occur in persons with chronic HBV infection. How is the virus spread? The hepatitis D virus is spread by exposure to blood and serous body fluids, contaminated needles and syringes, and via sexual transmission. What are the symptoms? Onset of hepatitis D is usually sudden. Symptoms include tiredness, nausea, vomiting, fever, stomach pain, tea-colored urine, and yellowing of the skin and eyes (jaundice). Hepatitis D infection in someone with chronic hepatitis B may be misdiagnosed as a worsening of chronic hepatitis B. How soon do the symptoms appear? Symptoms occur approximately 2 - 8 weeks after infection. How long can an infected person spread the virus? A person can spread the virus as long as it remains in their blood. The highest risk of exposure occurs just before the onset of acute illness. How is hepatitis D diagnosed? A blood test is used to detect infection with the hepatitis D virus. Can a person get hepatitis D again? If antibodies develop, one infection with the hepatitis D virus protects a person from getting it again. -

Autoimmune Liver Disease: Overlap and Outliers
Modern Pathology (2007) 20, S15–S30 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Autoimmune liver disease: overlap and outliers Mary K Washington Department of Pathology, Vanderbilt University Medical Center, Nashville, TN, USA The three main categories of autoimmune liver disease are autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC); all are well-defined entities with diagnosis based upon a constellation of clinical, serologic, and liver pathology findings. Although these diseases are considered autoimmune in nature, the etiology and possible environmental triggers of each remain obscure. The characteristic morphologic patterns of injury are a chronic hepatitis pattern of injury with prominent plasma cells in AIH, destruction of small intrahepatic bile ducts and canals of Hering in PBC, and periductal fibrosis and inflammation involving larger bile ducts with variable small duct damage in PSC. Serological findings include the presence of antimitochondrial antibodies in PBC, antinuclear, anti-smooth muscle, and anti-LKM antibodies in AIH, and pANCA in PSC. Although most cases of autoimmune liver disease fit readily into one of these three categories, overlap syndromes (primarily of AIH with PBC or PSC) may comprise up to 10% of cases, and variant syndromes such as antimitochondrial antibody-negative PBC also occur. Sequential syndromes with transition from one form of autoimmune liver disease to another are rare. Modern Pathology (2007) 20, S15–S30. doi:10.1038/modpathol.3800684 Keywords: autoimmune liver disease; autoimmune hepatitis; primary biliary cirrhosis; primary sclerosing cholangitis; overlap syndrome The three major categories of autoimmune liver Epidemiology and Demographic Features disease are autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing The worldwide prevalence of AIH is unknown; most cholangitis (PSC). -

Hepatitis A, Acute
Hepatitis A, Acute *NOTE-HIGHLIGHTED SECTION HAS BEEN REVISED IMMEDIATELY REPORTABLE DISEASE Per NJAC 8:57, healthcare providers and administrators shall immediately report by telephone confirmed and suspected cases of acute hepatitis A to the health officer of the jurisdiction where the ill or infected person lives, or if unknown, wherein the diagnosis is made. The health officer (or designee) must immediately institute the control measures listed below in section 6, “Controlling Further Spread,” regardless of weekend, holiday, or evening schedules. A directory of local health departments in New Jersey is available at http://www.state.nj.us/health/lh/directory/lhdselectcounty.shtml If the health officer is unavailable, the healthcare provider or administrator shall make the report to the Department by telephone to 609.826.5964, between 8:00 A.M. and 5:00 P.M. on non-holiday weekdays or to 609.392.2020 during all other days and hours. June 2019 Hepatitis A, Acute 1 THE DISEASE AND ITS EPIDEMIOLOGY A. Etiologic Agent Hepatitis A is caused by the hepatitis A virus (HAV), a ribonucleic acid (RNA) agent classified as a Picornavirus. There is only one serotype worldwide; it is slow-growing in living cells and resistant to heat, solvents, and acid. Depending on conditions, HAV can be stable in the environment for months. Heating foods at temperatures greater than 185°F (>85°C) for one minute or disinfecting surfaces with a 1:100 dilution of sodium hypochlorite (i.e., household bleach) in tap water is necessary to inactivate HAV. The major site of viral replication is in the liver. -

Diagnosis and Management of Autoimmune Hemolytic Anemia in Patients with Liver and Bowel Disorders
Journal of Clinical Medicine Review Diagnosis and Management of Autoimmune Hemolytic Anemia in Patients with Liver and Bowel Disorders Cristiana Bianco 1 , Elena Coluccio 1, Daniele Prati 1 and Luca Valenti 1,2,* 1 Department of Transfusion Medicine and Hematology, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (C.B.); [email protected] (E.C.); [email protected] (D.P.) 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected]; Tel.: +39-02-50320278; Fax: +39-02-50320296 Abstract: Anemia is a common feature of liver and bowel diseases. Although the main causes of anemia in these conditions are represented by gastrointestinal bleeding and iron deficiency, autoimmune hemolytic anemia should be considered in the differential diagnosis. Due to the epidemiological association, autoimmune hemolytic anemia should particularly be suspected in patients affected by inflammatory and autoimmune diseases, such as autoimmune or acute viral hepatitis, primary biliary cholangitis, and inflammatory bowel disease. In the presence of biochemical indices of hemolysis, the direct antiglobulin test can detect the presence of warm or cold reacting antibodies, allowing for a prompt treatment. Drug-induced, immune-mediated hemolytic anemia should be ruled out. On the other hand, the choice of treatment should consider possible adverse events related to the underlying conditions. Given the adverse impact of anemia on clinical outcomes, maintaining a high clinical suspicion to reach a prompt diagnosis is the key to establishing an adequate treatment. Keywords: autoimmune hemolytic anemia; chronic liver disease; inflammatory bowel disease; Citation: Bianco, C.; Coluccio, E.; autoimmune disease; autoimmune hepatitis; primary biliary cholangitis; treatment; diagnosis Prati, D.; Valenti, L. -

A Novel Method to Rescue and Culture Duck Astrovirus Type 1 in Vitro
Zhang et al. Virology Journal (2019) 16:112 https://doi.org/10.1186/s12985-019-1218-5 RESEARCH Open Access A novel method to rescue and culture duck Astrovirus type 1 in vitro Ruihua Zhang1,2†, Jingjing Lan1,2†, Haie Li1†, Junhao Chen1,2, Yupeng Yang1,2, Shaoli Lin1, Zhijing Xie1,2 and Shijin Jiang1,2* Abstract Background: Reverse genetics systems enable the manipulation of viral genomes and therefore serve as robust reverse genetic tools to study RNA viruses. A DNA-launched rescue system initiates the transcription of viral genomic cDNA from eukaryotic promoter in transfected cells, generating homogenous RNA transcripts in vitro and thus enhancing virus rescue efficiency. As one of the hazardous pathogens to ducklings, the current knowledge of the pathogenesis of duck astrovirus type 1 (DAstV-1) is limited. The construction of a DNA- launched rescue system can help to accelerate the study of the virus pathogenesis. However, there is no report of such a system for DAstV-1. Methods: In this study, a DNA-launched infectious clone of DAstV-1 was constructed from a cDNA plasmid, which contains a viral cDNA sequence flanked by hammerhead ribozyme (HamRz) and a hepatitis delta virus ribozyme (HdvRz) sequence at both terminals of the viral genome. A silent nucleotide mutation creating a Bgl II site in the ORF2 gene was made to distinguish the rescued virus (rDAstV-1) from the parental virus (pDAstV-1). Immunofluorescence assay (IFA) and western blot were conducted for rescued virus identification in duck embryo fibroblast (DEF) cells pre-treated with trypsin. The growth characteristics of rDAstV-1 and pDAstV-1 in DEF cells and the tissue tropism in 2-day-old ducklings of rDAstV-1 and pDAstV-1 were determined. -
Viral Hepatitis B: Introduction
Viral Hepatitis B: Introduction “Viral hepatitis," refers to infections that affect the liver and are caused by viruses. It is a major public health issue in the United States and worldwide. Not only does viral hepatitis carry a high morbidity, but it also stresses medical resources and can have severe economic consequences. The majority of all viral hepatitis cases are preventable. Viral hepatitis includes five distinct disease entities, which are caused by at least five different viruses. Hepatitis A and hepatitis B (infectious and serum hepatitis, respectively) are considered separate diseases and both can be diagnosed by a specific serologic test. Hepatitis C and E comprise a third category, each a distinct type, with Hepatitis C parenterally transmitted, and hepatitis E enterically transmitted. Hepatitis D, or delta hepatitis, is another distinct virus that is dependent upon hepatitis B infection. This form of hepatitis may occur as a super-infectionin a hepatitis B carrier or as a co-infection in an individual with acute hepatitis B. Hepatitis viruses most often found in the United States include A, B, C, and D. Because fatality from hepatitis is relatively low, mortality figures are a poor indicator of the actual incidence of these diseases. The Centers for Disease Control and Prevention estimated that approximately 400,000–600,000 people were infected with viral hepatitis during the decade of the 1990s. Hepatitis plagued mankind as early as the fifth century BC. It was referenced in early biblical literature and described as occurring in outbreaks, especially during times of war. Toward the end of the nineteenth century, hepatitis was thought to occur as a result of infection of the hepatic parenchyma. -

Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases 1 2 3 4 5 Keith D
| PRACTICE GUIDANCE HEPATOLOGY, VOL. 0, NO. 0, 2018 Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases 1 2 3 4 5 Keith D. Lindor, Christopher L. Bowlus, James Boyer, Cynthia Levy, and Marlyn Mayo Intended for use by health care providers, this guid- Preamble ance identifies preferred approaches to the diagnostic This American Association for the Study of and therapeutic aspects of care for patients with PBC. Liver Diseases (AASLD) 2018 Practice Guidance As with clinical practice guidelines, it provides gen- on Primary Biliary Cholangitis (PBC) is an update eral guidance to optimize the care of the majority of of the PBC guidelines published in 2009. The 2018 patients and should not replace clinical judgment for updated guidance on PBC includes updates on etiol- a unique patient. ogy and diagnosis, role of imaging, clinical manifesta- The major changes from the last guideline to this tions, and treatment of PBC since 2009. The AASLD guidance include information about obeticholic acid 2018 PBC Guidance provides a data-supported (OCA) and the adaptation of the guidance format. approach to screening, diagnosis, and clinical man- agement of patients with PBC. It differs from more recent AASLD practice guidelines, which are sup- Etiology of Primary Biliary ported by systematic reviews and a multidisciplinary panel of experts that rates the quality (level) of the Cholangitis evidence and the strength of each recommendation Primary Biliary Cholangitis (PBC) is considered using the Grading of Recommendations Assessment, an autoimmune disease because of its hallmark sero- Development, and Evaluation system. In contrast, this logic signature, antimitochondrial antibody (AMA), (1-4) guidance was developed by consensus of an expert and specific bile duct pathology.