Viral Hepatitis Testing Effective Date: January 1, 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

The Use of Non-Human Primates in Research in Primates Non-Human of Use The
The use of non-human primates in research The use of non-human primates in research A working group report chaired by Sir David Weatherall FRS FMedSci Report sponsored by: Academy of Medical Sciences Medical Research Council The Royal Society Wellcome Trust 10 Carlton House Terrace 20 Park Crescent 6-9 Carlton House Terrace 215 Euston Road London, SW1Y 5AH London, W1B 1AL London, SW1Y 5AG London, NW1 2BE December 2006 December Tel: +44(0)20 7969 5288 Tel: +44(0)20 7636 5422 Tel: +44(0)20 7451 2590 Tel: +44(0)20 7611 8888 Fax: +44(0)20 7969 5298 Fax: +44(0)20 7436 6179 Fax: +44(0)20 7451 2692 Fax: +44(0)20 7611 8545 Email: E-mail: E-mail: E-mail: [email protected] [email protected] [email protected] [email protected] Web: www.acmedsci.ac.uk Web: www.mrc.ac.uk Web: www.royalsoc.ac.uk Web: www.wellcome.ac.uk December 2006 The use of non-human primates in research A working group report chaired by Sir David Weatheall FRS FMedSci December 2006 Sponsors’ statement The use of non-human primates continues to be one the most contentious areas of biological and medical research. The publication of this independent report into the scientific basis for the past, current and future role of non-human primates in research is both a necessary and timely contribution to the debate. We emphasise that members of the working group have worked independently of the four sponsoring organisations. Our organisations did not provide input into the report’s content, conclusions or recommendations. -

Rational Engineering of HCV Vaccines for Humoral Immunity
viruses Review To Include or Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity Felicia Schlotthauer 1,2,†, Joey McGregor 1,2,† and Heidi E Drummer 1,2,3,* 1 Viral Entry and Vaccines Group, Burnet Institute, Melbourne, VIC 3004, Australia; [email protected] (F.S.); [email protected] (J.M.) 2 Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC 3000, Australia 3 Department of Microbiology, Monash University, Clayton, VIC 3800, Australia * Correspondence: [email protected]; Tel.: +61-392-822-179 † These authors contributed equally to this work. Abstract: Direct-acting antiviral agents have proven highly effective at treating existing hepatitis C infections but despite their availability most countries will not reach the World Health Organization targets for elimination of HCV by 2030. A prophylactic vaccine remains a high priority. Whilst early vaccines focused largely on generating T cell immunity, attention is now aimed at vaccines that gen- erate humoral immunity, either alone or in combination with T cell-based vaccines. High-resolution structures of hepatitis C viral glycoproteins and their interaction with monoclonal antibodies isolated from both cleared and chronically infected people, together with advances in vaccine technologies, provide new avenues for vaccine development. Keywords: glycoprotein E2; vaccine development; humoral immunity Citation: Schlotthauer, F.; McGregor, J.; Drummer, H.E To Include or 1. Introduction Occlude: Rational Engineering of HCV Vaccines for Humoral Immunity. The development of direct-acting antiviral agents (DAA) with their ability to cure Viruses 2021, 13, 805. https:// infection in >95% of those treated was heralded as the key to eliminating hepatitis C globally. -

Research Directions After the First Hepatitis C Vaccine Efficacy Trial
viruses Review Where to Next? Research Directions after the First Hepatitis C Vaccine Efficacy Trial Christopher C. Phelps 1 , Christopher M. Walker 1,2 and Jonathan R. Honegger 1,2,*,† 1 Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH 43205, USA; [email protected] (C.C.P.); [email protected] (C.M.W.) 2 Department of Pediatrics, The Ohio State University College of Medicine, Columbus, OH 43210, USA * Correspondence: [email protected] † Current address: Abigail Wexner Research Institute at Nationwide Children’s Hospital, 700 Children’s Drive, WA4020, Columbus, OH 43205, USA. Abstract: Thirty years after its discovery, the hepatitis C virus (HCV) remains a leading cause of liver disease worldwide. Given that many countries continue to experience high rates of transmission despite the availability of potent antiviral therapies, an effective vaccine is seen as critical for the elimination of HCV. The recent failure of the first vaccine efficacy trial for the prevention of chronic HCV confirmed suspicions that this virus will be a challenging vaccine target. Here, we examine the published data from this first efficacy trial along with the earlier clinical and pre-clinical studies of the vaccine candidate and then discuss three key research directions expected to be important in ongoing and future HCV vaccine development. These include the following: 1. design of novel immunogens that generate immune responses to genetically diverse HCV genotypes and subtypes, 2. strategies to elicit broadly neutralizing antibodies against envelope glycoproteins in addition to cytotoxic and helper T cell responses, and 3. -

All Qatar Residents to Get Covid Vaccine Free
INDEX QATAR 2-6,12 COMMENT 10 BUSINESS | Page 1 QATAR | Page 12 ARAB WORLD 7 BUSINESS 1-12 Qatar private INTERNATIONAL 7-9,11 SPORTS 1-8 Last Covid-19 sector bounces DOW JONES QE NYMEX patient back on lift ing discharged 28,148.64 9,956.66 39.36 of Covid-19 +465.83 +3.15 +2.31 from Lebsear +1.68% +0.03% +6.23% curbs: QFC Latest Figures Field Hospital published in QATAR since 1978 TUESDAY Vol. XXXXI No. 11693 October 6, 2020 Safar 19, 1442 AH GULF TIMES www. gulf-times.com 2 Riyals PM offers condolences to Amir of Kuwait HE the Prime Minister and Minister of Interior Sheikh Khalid bin Khalifa bin Abdulaziz al-Thani yesterday off ered condolences to the Amir of Kuwait, Sheikh Nawaf al-Ahmad al-Jaber al-Sabah, on the death of Sheikh Sabah al-Ahmad al-Jaber al-Sabah, praying to Allah to have mercy on the soul of the deceased and to rest it in peace in Paradise. The prime minister also off ered condolences to members of the ruling family and ranking off icials. Ministers and members of the off icial delegation accompanying the prime minister also off ered their condolences. Two held for violating PM meets Afghan president home quarantine rules Competent authorities arrested yesterday two people who violated All Qatar residents to the requirements of the home quarantine, which they committed to following, in accordance with the procedures of the health authorities in the country. The get Covid vaccine free two persons being referred to prosecution are: Albert Mondano Oshavillo and Nasser Ghaidan By Ayman Adly is not yet clear whether the upcoming Mohamed al-Hatheeth al-Qahtani. -
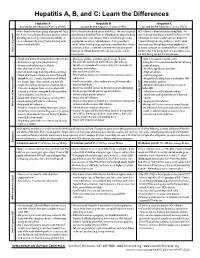
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant
medRxiv preprint doi: https://doi.org/10.1101/2021.05.14.21257248; this version posted May 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant-Produced Virus-Like Particle 2 Vaccine for Covid-19 in Healthy Adults Aged 18-64 and Older Adults Aged 65 and Older 3 Authors: Philipe Gobeil1, Stéphane Pillet1, Annie Séguin1, Iohann Boulay1, Asif Mahmood1, 4 Donald C Vinh 2, Nathalie Charland1, Philippe Boutet3, François Roman3, Robbert Van Der 5 Most4, Maria de los Angeles Ceregido Perez3, Brian J Ward1,2†, Nathalie Landry1† 6 Affiliations: 1 Medicago Inc., 1020 route de l’Église office 600, Québec, QC, Canada, G1V 7 3V9; 2 Research Institute of the McGill University Health Centre, 1001 Decarie St, Montreal, 8 QC H4A 3J1; 3 GlaxoSmithKline Biologicals SA (Vaccines), Avenue Fleming 20, 1300 Wavre, 9 Belgium; 4 GlaxoSmithKline Biologicals SA (Vaccines), rue de l’Institut 89, 1330 Rixensart, 10 Belgium; † These individuals are equally credited as senior authors. 11 * Corresponding author: Nathalie Landry, 1020 Route de l’Église, Bureau 600, Québec, Qc, 12 Canada, G1V 3V9; Tel. 418 658 9393; Fax. 418 658 6699; [email protected] 13 Abstract 14 The rapid spread of SARS-CoV-2 globally continues to impact humanity on a global scale with 15 rising morbidity and mortality. Despite the development of multiple effective vaccines, new 16 vaccines continue to be required to supply ongoing demand. -

Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines
Review Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines Puna Maya Maharjan 1 and Sunghwa Choe 2,3,* 1 G+FLAS Life Sciences, 123 Uiryodanji-gil, Osong-eup, Heungdeok-gu, Cheongju-si 28161, Korea; punamaya.maharjan@gflas.com 2 G+FLAS Life Sciences, 38 Nakseongdae-ro, Gwanak-gu, Seoul 08790, Korea 3 School of Biological Sciences, College of Natural Sciences, Seoul National University, Gwanak-gu, Seoul 08826, Korea * Correspondence: [email protected] Abstract: The prevalence of the coronavirus disease 2019 (COVID-19) pandemic in its second year has led to massive global human and economic losses. The high transmission rate and the emergence of diverse SARS-CoV-2 variants demand rapid and effective approaches to preventing the spread, diagnosing on time, and treating affected people. Several COVID-19 vaccines are being developed using different production systems, including plants, which promises the production of cheap, safe, stable, and effective vaccines. The potential of a plant-based system for rapid production at a commercial scale and for a quick response to an infectious disease outbreak has been demonstrated by the marketing of carrot-cell-produced taliglucerase alfa (Elelyso) for Gaucher disease and tobacco- produced monoclonal antibodies (ZMapp) for the 2014 Ebola outbreak. Currently, two plant-based COVID-19 vaccine candidates, coronavirus virus-like particle (CoVLP) and Kentucky Bioprocessing (KBP)-201, are in clinical trials, and many more are in the preclinical stage. Interim phase 2 clinical Citation: Maharjan, P.M.; Choe, S. trial results have revealed the high safety and efficacy of the CoVLP vaccine, with 10 times more Plant-Based COVID-19 Vaccines: neutralizing antibody responses compared to those present in a convalescent patient’s plasma. -

Information & Consent Form
INFORMATION & CONSENT FORM RESEARCH TITLE: A Randomized, Observer-Blind, Placebo-Controlled, Phase 2/3 Study to Assess the Safety, Efficacy, and Immunogenicity of a Recombinant Coronavirus-Like Particle COVID-19 Vaccine in Adults 18 Years of Age or Older SHORT TITLE: A randomized controlled study, assessing the safety and immunogenicity of a COVID-19 vaccine in adults PROTOCOL NUMBER: CP-PRO-CoVLP-021 RESEARCHERS: Principal Investigator: Dr. Scott Halperin, Pediatric Infectious Disease Specialist, IWK Health Centre Co-Investigators: Dr. Joanne Langley, Pediatric Infectious Disease Specialist, IWK Health Centre Dr. Jeannette Comeau, Pediatric Infectious Disease Specialist, IWK Health Centre FUNDER: Medicago R&D Inc. SPONSOR: Medicago R&D Inc. 1020 route de l’Église, bureau 600 Québec (QC), Canada G1V 3V9 Introduction You are being asked to take part in this research study to assess the safety and ability of an investigational study vaccine to generate an immune response against the virus causing COVID-19 disease. You can decide if you want to take part in this study or not. Your choice will not change the quality of care that you will receive outside of this study. Please take time to read the following information about the study. Feel free to ask the study staff any questions that you may have. Informed consent means agreeing to take part in a research study, but only when you fully understand what this means for you. You sign this informed consent form only if you agree to take part in this study. Signing the form shows that you have understood what this means for you and that you want to take part. -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days. -

HEPATITIS D the Most Virulent Hepatitis Virus of All
HEPATITIS D The Most Virulent Hepatitis Virus Of All The hepatitis D virus is perhaps the most unique of all hepatitis viruses, and also the most virulent. As a virus, it is flawed. The hepatitis D virus (HDV) cannot replicate and infect someone unless the person is already infected with the hepatitis B virus (HBV). The hepatitis D virus requires the outer coating of the hepatitis B virus—called the surface antigen—in order to reproduce itself in a human host. The virus currently infects 15 million worldwide, nearly all adults, and it is most common among injecting drug user populations and in countries bordering the Mediterranean Sea. Most children with HDV infection live in Italy and Greece, with a few in northern Africa. There are no reports or estimates of There are two types of HDV infection, the number of children infected with coinfection and super-infection: HDV worldwide. • Coinfection occurs when a patient is Despite widespread hepatitis B in simultaneously infected with HDV and Asian populations, HDV infection is HBV. The majority of these patients virtually unknown there and appears completely recover but there is a higher rate of fulminant hepatic failure and primarily in injecting drug user death than with HBV infection alone. populations. • Super-infection occurs when someone HDV is transmitted through blood and with an existing chronic HBV infection body fluids, similar to the hepatitis B becomes infected with HDV. These patients usually experience a sudden virus. Hepatitis D symptoms are worsening of liver disease. Patients with similar to other viral hepatitis diseases hepatitis B who become chronically and include jaundice, fever, malaise, infected with HDV experience a very dark urine and nausea. -

HEPATITIS D (Viral Hepatitis D, Hepatitis Delta Virus, Delta Agent Hepatitis, Delta Associated Hepatitis)
FACT SHEET HEPATITIS D (Viral hepatitis D, Hepatitis delta virus, Delta agent hepatitis, Delta associated hepatitis) What is hepatitis D? Hepatitis D is a virus that infects the liver. Hepatitis D, or Delta hepatitis, is always associated with a hepatitis B infection. A person may recover from Delta hepatitis or it may progress to chronic hepatitis. Who gets hepatitis D? Hepatitis D can only occur if the person has hepatitis B. Hepatitis D virus (HDV) and hepatitis B virus (HBV) may infect a person at the same time or HDV infection may occur in persons with chronic HBV infection. How is the virus spread? The hepatitis D virus is spread by exposure to blood and serous body fluids, contaminated needles and syringes, and via sexual transmission. What are the symptoms? Onset of hepatitis D is usually sudden. Symptoms include tiredness, nausea, vomiting, fever, stomach pain, tea-colored urine, and yellowing of the skin and eyes (jaundice). Hepatitis D infection in someone with chronic hepatitis B may be misdiagnosed as a worsening of chronic hepatitis B. How soon do the symptoms appear? Symptoms occur approximately 2 - 8 weeks after infection. How long can an infected person spread the virus? A person can spread the virus as long as it remains in their blood. The highest risk of exposure occurs just before the onset of acute illness. How is hepatitis D diagnosed? A blood test is used to detect infection with the hepatitis D virus. Can a person get hepatitis D again? If antibodies develop, one infection with the hepatitis D virus protects a person from getting it again.