Viral Hepatitis a & E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Viral Hepatitis Testing Effective Date: January 1, 2012
Viral Hepatitis Testing Effective Date: January 1, 2012 Scope This guideline provides guidance for the use of laboratory tests to diagnose acute and chronic viral hepatitis in adults (> 19 years) in the primary care setting. General Considerations for Ordering Laboratory Tests Prior to ordering tests for hepatitis, consider the patient’s history, age, risk factors (see below), hepatitis vaccination status, and any available previous hepatitis test results. Risk Factors for Viral Hepatitis include: • Substance use (includes sharing drug snorting, smoking or injection equipment) • High-risk sexual activity or sexual partner with viral hepatitis • Travel to or from high-risk hepatitis endemic areas or exposure during a local outbreak • Immigration from hepatitis B and/or C endemic countries • Household contact with an infected person especially if personal items (e.g., razors, toothbrushes, nail clippers) are shared • Recipient of unscreened blood products* • Needle-stick injury or other occupational exposure (e.g., healthcare workers) • Children born to mothers with chronic hepatitis B or C infection • Attendance at daycare • Contaminated food or water (hepatitis A only) • Tattoos and body piercing • History of incarceration • HIV or other sexually transmitted infection • Hemodialysis *screening of donated blood products for hepatitis C (anti-HCV) began in 1990 in Canada.1 Types of Viral Hepatitis Hepatitis A: causes acute but not chronic hepatitis Hepatitis B: causes acute and chronic hepatitis Hepatitis C: causes chronic hepatitis but rarely manifests as acute hepatitis Hepatitis D: rare and only occurs in patients infected with hepatitis B Hepatitis E: clinically similar to hepatitis A, mostly restricted to endemic areas and occasionally causes chronic infection in immunosuppressed people Others: e.g. -
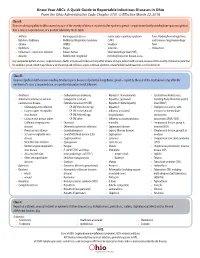
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -
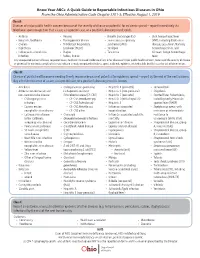
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

CAHAN San Diego Alert Template
To: CAHAN San Diego Participants Date: April 10, 2018 From: Public Health Services, Epidemiology and Immunizations Services Branch Update: Wound Botulism Cases Associated with Black Tar Heroin This health advisory updates providers about three recently reported wound botulism cases associated with black tar heroin use in San Diego County and provides recommendations on management. Three cases of wound botulism in local heroin users were reported in 2017, two of which were described in a previous CAHAN. Situation In the past month, two confirmed cases and one highly suspect case of wound botulism associated with black tar heroin injection have been reported in San Diego County. The cases are all male, range in age from 28 to 67, and apparently are unknown to each other. They presented with wound infections or abscesses and a recent history of skin or muscle popping black tar heroin. Other symptoms included diplopia, ptosis, extraocular palsy, dysphagia, slurred speech, and generalized weakness. All required intensive care treatment, and two have had respiratory failure requiring intubation. All patients were treated with Botulism Antitoxin Heptavalent (BAT®) released by the California Department of Public Health (CDPH). The sources of the black tar heroin remain unknown and additional cases may occur. Clusters of botulism cases associated with black tar heroin injection have occurred in Southern California in the past, including five cases in San Diego County in 2010. Background Botulism is a rare and potentially fatal illness caused by the neurotoxin produced by Clostridium botulinum and rarely by other Clostridia species. Routes of exposure vary: patients may present with wound botulism, commonly associated with injection drug use; with foodborne botulism from ingestion of contaminated food items; with infant botulism (the intestinal toxemia form of botulism in patients ≤15months of age); and, rarely, with adult intestinal botulism, or with an iatrogenic exposure to therapeutic botulinum toxin. -

Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom
LETTERS term to dramatically reduce the high 3. Biswas PK, Christensen JP, Ahmed SS, biologically relevant sentinels and incidence of HPAI in Bangladesh. We Barua H, Das A, Rahman MH. at al. Avian can indicate potential pathogens that infl uenza outbreaks in chickens, Bangla- have progressively and dramatically desh. Emerg Infect Dis. 2008;14:1909–12. are contaminating the environment. increased the scope and benefi ts of our http://dx.doi.org/10.3201/eid1412.071567 It is essential to ensure that this pilot PVC implementation program, 4. Biswas PK, Christensen JP, Ahmed SS, sustainable resource of coastal areas, but additional work is needed. To Das A, Rahman MH, Barua H, et al. where mussels and oysters are farmed Risk for infection with highly pathogenic help spread PVC approaches through- avian infl uenza virus (H5N1) in back- or collected wild, is not subjected to out the country, community leaders, yard chickens, Bangladesh. Emerg In- environmental contamination that imams of local mosques, and school fect Dis. 2009;15:1931–6. http://dx.doi. could lead to public health risks. teachers can be trained to implement org/10.3201/eid1512.090643 Risk management for bivalve 5. Biswas PK, Rahman MH, Das A, Ahmed awareness programs on safe practices SS, Giasuddin M, Christensen JP. Risk mollusks, aimed at control of fecal for raising poultry and regular clean- for highly pathogenic avian infl uenza pollution, relies heavily on the use ing and disinfection of live bird mar- H5N1 virus infection in chickens in of Escherichia coli as an indicator kets. The strengthening of biosecurity small-scale commercial farms, in a high- of fecal (sewage) contamination risk area, Bangladesh, 2008. -
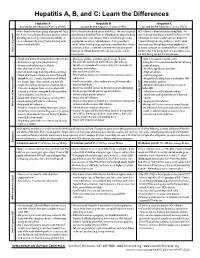
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Emerging Water-Borne Pathogens
Appl Microbiol Biotechnol (2003) 61:424–428 DOI 10.1007/s00253-003-1302-y MINI-REVIEW S. Sharma · P. Sachdeva · J. S. Virdi Emerging water-borne pathogens Received: 1 October 2002 / Revised: 27 February 2003 / Accepted: 28 February 2003 / Published online: 9 April 2003 Springer-Verlag 2003 Abstract Emerging water-borne pathogens constitute a number of reviews and other publications available on the major health hazard in both developed and developing subject discuss emerging and re-emerging pathogens in nations. A new dimension to the global epidemiology of general, including water-borne, without addressing the cholera—an ancient scourge—was provided by the issues specifically pertinent to emerging water-borne emergence of Vibrio cholerae O139. Also, water-borne pathogens. This review critically examines which organ- enterohaemorrhagic Escherichia coli (E. coli O157:H7), isms really qualify as emerging water-borne pathogens, although regarded as a problem of the industrialized west, the possible reasons underlying their emergence and has recently caused outbreaks in Africa. Outbreaks of specific measures to face the challenge posed by them. chlorine-resistant Cryptosporidium have motivated water Due to the paucity of data, it may be difficult to decide authorities to reassess the adequacy of current water- which of all water-borne pathogens are emerging. Nev- quality regulations. Of late, a host of other organisms, ertheless, there are some clear-cut candidates. such as hepatitis viruses (including hepatitis E virus), No organism other than Vibrio cholerae could serve as Campylobacter jejuni, microsporidia, cyclospora, Yersin- a better example of an emerging water-borne pathogen. ia enterocolitica, calciviruses and environmental bacteria Cholera is an ancient scourge and to date seven like Mycobacterium spp, aeromonads, Legionella pneu- pandemics have been recorded. -

Disease Reporting-Clinician
Infections, diseases and conditions reportable by clinicians: 2015 Report immediately • Anthrax (Bacillus anthracis) • Botulism (Clostridium botulinum) • Cholera (Vibrio cholerae O1, O139, or toxigenic) • Diphtheria (Corynebacterium diphtheriae) • Hemorrhagic fever caused by viruses of the filovirus (e.g., Ebola, Marburg) or arenavirus (e.g., Lassa, Machupo) families • Influenza (novel)1 (From footnote: Influenza A virus that cannot be subtyped by commercially distributed assays) • Marine intoxication (intoxication caused by marine microorganisms or their byproducts (e.g., paralytic shellfish poisoning, domoic acid intoxication, ciguatera, scombroid) • Measles (rubeola) • Plague (Yersinia pestis) • Poliomyelitis • Rabies (human) • Rubella • SARS (Severe Acute Respiratory Syndrome or SARS-coronavirus) • Smallpox (variola) • Tularemia (Francisella tularensis) • Yellow fever • Outbreaks and uncommon illnesses (any known or suspected common-source outbreak; any uncommon illness of potential public health significance) Report within 24 hours (including weekends and holidays) • Haemophilus influenzae (any isolation or identification from a normally sterile specimen type) • Neisseria meningitidis • Pesticide poisoning Public Health Division | Infections, diseases and conditions reportable by clinicians 128 Report within one working day • Amebic infections (central nervous system only) (for example infection by Naegleria or Balamuthia spp. • Animal bites (of humans) • Arthropod vector-borne disease (babesiosis, California encephalitis, Colorado -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days.