UNIVERSITY of CALIFORNIA Los Angeles Onsite Defluoridation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of New Low Cost Defluoridation Technology (Krass)
257 99DE Pergamon Wai Sci. Tech. Vol. 40, No. 2, pp. 167-173, 1999 © 1999 IAWQ Published by Elsevicr Science Ltd Printed in Great Britain. All rights reserved «273-1223/99 S20.00 + 000 PII: S0273-1223(99)00440-0 DEVELOPMENT OF NEW LOW COST DEFLUORIDATION TECHNOLOGY (KRASS) Kailash C. Agarwal*, Sunil K. Gupta** and Akhilendra B. Gupta* * Department of Civil Engineering. Malavjya Regional Engineering College, Jaipur 302 017, India ** Satellite Hospital, Bani Park. Jaipur 302 001, India ABSTRACT Systemic tluorosis is an endemic problem in several developing countries. In India 15 states are endemic for fluorosis, of which 5 have indicated hyperendemicity for fluorosts in all districts. WHO standards permit only 1 mg/1 as a safe limit for human consumption. People in several districts of Rajasthan are forced to consume water with fluoride concentrations of up to 44 mg/1 which has resulted in permanent deformities, joint pains, general debility and misery. About 60% of fluoride intake is through water. Considerable work on fluoride removal from water has been done all over the world. However a safe, efficient, free from residual aluminium in treated water, and cost effective defluoridation technique/process is not available and needs to be developed in order to prevent the occurrence of fluorosis. This paper describes the development of a defluoridation process which differs from the known processes in its simplicity, cost effectiveness and results in traces of residual aluminium in treated water. The parameters like fluoride concentration, temperature, pH, alkalinity, humidity and total dissolved solids of input water do not affect this process. -

Experiences with Domestic Defluoridation in India
DAW 30th WEDC International Conference, Vientiane, Lao PDR, 2004 PEOPLE-CENTRED APPROACHES TO WATER AND ENVIRONMENTAL SANITATION Experiences with domestic defl uoridation in India R.K. Daw During the International Drinking Water Supply and Sanitation Decade 1980-1990 in India, there was a marked preference in rural drinking water supply programmes, to seek community based water treatment solutions, attached to handpumps where such problems existed. This was true for iron removal and defl uoridation. However, over the next few years most of these treatments systems became inoperative, primarily attributed to a lack of a sense of ownership by user communities, resulting in an attitude of indifference towards the operation and maintenance of these plants. It is also possible that the complexities of O&M by user communities, in the rural Indian socio-political context, had not been institutionally under- stood. Since 1990s there has been an increasing trend to seek household based water quality treatment solution within rural drinking water supply programmes in India. This paper summarises the experiences of UNICEF in India in tackling the problem of high fl uoride content in rural drinking water supply sources, using household-based defl uoridation fi lter using activated alumina. Since 1991, UNICEF supported the research work for development of the technology by the Depart- ment of Chemistry, Indian Institute of Technology (IIT), Kanpur. This resulted in pilot projects on Domestic Defl uoridation Units in the states of Andhra Pradesh and Rajasthan during 1996-2002. Gradually a demand for these fi lters has grown and the private sector is gradually becoming interested. -

CAHAN San Diego Alert Template
To: CAHAN San Diego Participants Date: April 10, 2018 From: Public Health Services, Epidemiology and Immunizations Services Branch Update: Wound Botulism Cases Associated with Black Tar Heroin This health advisory updates providers about three recently reported wound botulism cases associated with black tar heroin use in San Diego County and provides recommendations on management. Three cases of wound botulism in local heroin users were reported in 2017, two of which were described in a previous CAHAN. Situation In the past month, two confirmed cases and one highly suspect case of wound botulism associated with black tar heroin injection have been reported in San Diego County. The cases are all male, range in age from 28 to 67, and apparently are unknown to each other. They presented with wound infections or abscesses and a recent history of skin or muscle popping black tar heroin. Other symptoms included diplopia, ptosis, extraocular palsy, dysphagia, slurred speech, and generalized weakness. All required intensive care treatment, and two have had respiratory failure requiring intubation. All patients were treated with Botulism Antitoxin Heptavalent (BAT®) released by the California Department of Public Health (CDPH). The sources of the black tar heroin remain unknown and additional cases may occur. Clusters of botulism cases associated with black tar heroin injection have occurred in Southern California in the past, including five cases in San Diego County in 2010. Background Botulism is a rare and potentially fatal illness caused by the neurotoxin produced by Clostridium botulinum and rarely by other Clostridia species. Routes of exposure vary: patients may present with wound botulism, commonly associated with injection drug use; with foodborne botulism from ingestion of contaminated food items; with infant botulism (the intestinal toxemia form of botulism in patients ≤15months of age); and, rarely, with adult intestinal botulism, or with an iatrogenic exposure to therapeutic botulinum toxin. -

Technologies for Fluoride Removal
22 Technologies for fluoride removal Leela Lyengar 22 Tec hnologies for fluoride removal 22.1. Introduction Fluoride is a normal constituent of natural water samples. Its concentration, though, varies significantly depending on the water source. Although both geological and man- made sources contribute to the occurrence of fluoride in water, the major contribution comes from geological resources. Except in isolated cases, surface waters seldom have fluoride levels exceeding 0.3 mg/l. Examples are streams flowing over granite rich in fluoride minerals and rivers that receive untreated fluoride-rich industrial wastewater. There are several fluoride bearing minerals in the earth's crust. They occur in sedimentary (limestone and sandstone) and igneous (granite) rocks. Weathering of these minerals along with volcanic and fumarolic processes lead to higher fluoride levels in groundwater. Dissolution of these barely soluble minerals depends on the water composition and the time of contact between the source minerals and the water. Over the years groundwater has generally been considered to be a protected and safe source of water, fit for drinking without treatment, as the main focus has been on the bacteriological quality of potable water. Little consideration used to be given to the risks of chemical pollution, particularly to the presence of elevated levels of fluoride, arsenic and nitrate in groundwater. This chapter deals with only fluoride. Consumption of water having excess fluoride over a prolonged period leads to a chronic ailment known as fluorosis. Incidence of high-fluoride groundwater has been reported from 23 nations around the globe. It has led to endemic fluorosis, which has become a major geo- environmental health issue in many developing countries. -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days. -

2 Model Forms, and Other Aids
ANNEX 2 Model Forms, and Other Aids 1. Applicant / Food Employee Interview Form 2. Applicant / Food Employee Reporting Agreement 3. Applicant / Food Employee Medical Referral Form 1 Applicant/Food Employee Interview Form Preventing Transmission of Diseases through Food by Infected Food Employees with Emphasis on illness due to Salmonella Typhi, Shigella spp., Escherichia coli 0157:H7, and Hepatitis A Virus The purpose of this form is to ensure that Applicants to whom a conditional offer of employment has been made and Food Employees advise the Person in Charge of past and current conditions described so that Person in Charge can take appropriate steps to preclude the transmission of foodborne illness. Applicant / Food Employee Name: (print)____________________________________________________________ Address: _______________________________________________________________________________________ _____________________________________________________________________________________________________________ Telephone: Daytime: _________________________ Evening: _________________________ TODAY: Are you now suffering from any of the following: 1. Symptoms Diarrhea? YES / NO Fever? YES / NO Vomiting? YES / NO Jaundice? YES / NO Sore throat with fever? YES / NO 2. Lesions containing pus on the hand, wrist or exposed body part? (such as boils and infected wounds, however small) YES / NO PAST: Have you ever been exposed to or suspected of causing a confirmed outbreak of typhoid fever (Salmonella Typhi), shigellosis (Shigella spp.), Escherichia coli O157:H7 infection (E. coli 0157:H7), or Hepatitis A (hepatitis A virus)? YES / NO If you have, what was the date of the diagnosis?_____________________________ HIGH-RISK CONDITIONS: 1. Have you been exposed to or suspected of causing a confirmed outbreak of typhoid fever, shigellosis, E. coli O157:H7 infection, or hepatitis A? YES / NO 2. Do you live in the same household as a person diagnosed with typhoid fever, shigellosis, hepatitis A, or illness due to E. -

The Big Five Foodborne Illnesses
The Big Five Foodborne Illnesses: E. Coli Overview: A bacterium that can produce a deadly toxin and causes approximately 73,000 cases of foodborne illness each year in the U.S.; Sources: meat, especially undercooked or raw hamburger, produce and raw milk Incubation Period: 3-4 days Symptoms: Severe diarrhea, cramping, dehydration Prevention: Cook implicated food to 155F; wash hands properly & frequently; correctly wash, rinse & sanitize dishware Shigella Overview: Causes an estimated 300,000 cases of diarrhea illnesses. Poor hygiene causes Shigella to be easily passed from person to person. Sources: salads, milk and dairy products, and unclean water. Incubation Period: 1-7 days Symptoms: Diarrhea, fever, chills, and dehydration Prevention: Wash hands frequently and properly, especially after using the restroom; wash vegetables thoroughly Hepatitis A Overview: Hepatitis A is a liver disease caused by the hepatitis A virus. Hepatitis A can affect anyone. In the United States, hepatitis A can occur in situations ranging from isolated cases of disease to widespread epidemics. Incubation Period: 15-50 days Symptoms: Jaundice, nausea, diarrhea, fever, fatigue, loss of appetite, cramps Prevention: Wash hands frequently and properly, especially after using the restroom Salmonella Overview: Most common cause of foodborne deaths. Responsible for millions of cases of foodborne illness a year; Sources: raw and undercooked eggs, undercooked poultry and meat, dairy products, seafood, fruits and vegetables Incubation Period: 5-72 hours Symptoms: Nausea, vomiting, cramps, and fever Prevention: Cook all food to proper temperatures; chill food rapidly; eliminate sources of cross contamination (i.e. proper meat storage, proper wash, rinse, and sanitize procedure) Norovirus Overview: This virus is the leading cause of diarrhea in the United States. -

Treatment of Fluoride-Contaminated Water. a Review
Treatment of fluoride-contaminated water. A review P Senthil Kumar, S Suganya, S Srinivas, S Priyadharshini, M Karthika, R Karishma Sri, V Swetha, Mu Naushad, Eric Lichtfouse To cite this version: P Senthil Kumar, S Suganya, S Srinivas, S Priyadharshini, M Karthika, et al.. Treatment of fluoride- contaminated water. A review. Environmental Chemistry Letters, Springer Verlag, 2019, 17 (4), pp.1707 - 1726. 10.1007/s10311-019-00906-9. hal-02403012 HAL Id: hal-02403012 https://hal.archives-ouvertes.fr/hal-02403012 Submitted on 10 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Environmental Chemistry Letters (2019) 17:1707–1726 https://doi.org/10.1007/s10311-019-00906-9 Revised version REVIEW Treatment of fuoride‑contaminated water. A review P. Senthil Kumar1 · S. Suganya1 · S. Srinivas1 · S. Priyadharshini1 · M. Karthika1 · R. Karishma Sri1 · V. Swetha1 · Mu. Naushad2 · Eric Lichtfouse3 Abstract Delivering the right amount of fuoride to drinking water protects the teeth from decay and reduces the risk of cavities. Nonetheless, fuorosis has been diagnosed as the result of excessive exposure of fuoride, which induces brain impairment, muscle disorders and hyperactivity. Fluoride ingestion during the formation of the tooth enamel is the main reason for fuo- rosis, which is characterized by hypomineralization. -
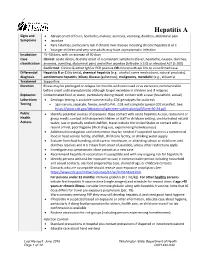
Hepatitis a Reporting Guideline
Hepatitis A Signs and • Abrupt onset of fever, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain Symptoms • Jaundice • Rare fatalities, particularly risk if chronic liver disease including chronic hepatitis B or C • Younger children and very rare adults may have asymptomatic infection Incubation 15–50 days, with an average of 30 days Case Clinical: acute illness, discrete onset of a consistent symptoms (fever, headache, nausea, diarrhea, classification anorexia, vomiting, abdominal pain) and either jaundice (bilirubin ≥ 3.0) or elevated ALT (≥ 200) Confirmed: Clinical & either IgM or PCR positive OR clinical with epi link to a confirmed case Differential Hepatitis B or C (do tests), chemical hepatitis (e.g., alcohol, some medications, natural products), diagnosis autoimmune hepatitis, biliary disease (gallstones), malignancy, metabolic (e.g., Wilson’s) Treatment Supportive Duration Illness may be prolonged or relapse for months with continued virus excretion; communicable before onset until asymptomatic although longer excretion in children and if relapses Exposures Contaminated food or water, particularly during travel; contact with a case (household, sexual) Laboratory • Serologic testing is available commercially; CDC genotypes for outbreak Testing • Spin serum, separate, freeze, send to PHL. CDE will complete special CDC manifest. See: https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf. Public • Identify potential sources of exposure: close contact with acute hepatitis A case, restaurant or Health -

Guidelines for Drinking-Water Quality FIRST ADDENDUM to THIRD EDITION Volume 1 Recommendations WHO Library Cataloguing-In-Publication Data World Health Organization
Guidelines for Drinking-water Quality FIRST ADDENDUM TO THIRD EDITION Volume 1 Recommendations WHO Library Cataloguing-in-Publication Data World Health Organization. Guidelines for drinking-water quality [electronic resource] : incorporating first addendum. Vol. 1, Recommendations. – 3rd ed. Electronic version for the Web. 1.Potable water – standards. 2.Water – standards. 3.Water quality – standards. 4.Guidelines. I. Title. ISBN 92 4 154696 4 (NLM classification: WA 675) © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expres- sion of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Hepatitis a and Hepatitis E Luis S
Hepatitis A and Hepatitis E Luis S. Marsano, MD Professor of Medicine Division of Gastroenterology, Hepatology & Nutrition University of Louisville and Louisville VAMC 2011 Viruses that cause Hepatitis in Humans • Hepatitis A • Lassa virus • Hepatitis B • Rift Valley virus • Hepatitis C • Marburg virus • Hepatitis D • Ebola virus • Hepatitis E • Measles virus • Coxsackie • Human adenovirus • Echovirus • CMV • Yellow fever • EBV • Rubella • HSV • Junin virus (Argentina) • Varicella‐Zoster • Machupo virus (Bolivia) Hepatitis A Hepatitis A • Hepatovirus from Picornavirus family. • 27‐32 nm, linear, (+) sense, simple stranded RNA. • Reservoir: human only • Acquisition: fecal‐oral; concentrated in oysters. Contaminated water or food, men having sex with men, blood during short viremic phase. • Non‐cytopatic; immune mediated injury by lymphocytes Groups at Risk for HAV • Healthy persons who travel to endemic areas, • Workers in occupations with high likelihood of exposure, • Family members of infected patients, • Persons who adopt infants or children from endemic areas, • Men who have sex with men, • Persons who have tested positive for human immunodeficiency virus, • Persons with chronic liver disease, • Persons with clotting factor disorders, • Users of injection and noninjection illicit drugs. Hepatitis A Sero‐Prevalence • Poor sanitation countries: – near 100 % by age 5. • Good sanitation countries (USA) : – 10 % by age 14; – 37 % in adults. Hepatitis A Clinical Features • Incubation: 2‐4 (up to 6) weeks • Children < 2 year: 80% anicteric & asymptomatic • Children > 2 y and adults: 80% icteric & symptomatic • Symptoms: Fatigue (90%), anorexia (85%), jaundice (80%), nausea (75%), low fever (65%), headache, abdominal pain and myalgias. • Duration: usually < 8 weeks Hepatitis A Atypical Manifestations • Relapsing hepatitis: less than 10%; 2 or more bouts of elevated enzymes.