Guidelines for Viral Hepatitis Surveillance and Case Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAHAN San Diego Alert Template
To: CAHAN San Diego Participants Date: April 10, 2018 From: Public Health Services, Epidemiology and Immunizations Services Branch Update: Wound Botulism Cases Associated with Black Tar Heroin This health advisory updates providers about three recently reported wound botulism cases associated with black tar heroin use in San Diego County and provides recommendations on management. Three cases of wound botulism in local heroin users were reported in 2017, two of which were described in a previous CAHAN. Situation In the past month, two confirmed cases and one highly suspect case of wound botulism associated with black tar heroin injection have been reported in San Diego County. The cases are all male, range in age from 28 to 67, and apparently are unknown to each other. They presented with wound infections or abscesses and a recent history of skin or muscle popping black tar heroin. Other symptoms included diplopia, ptosis, extraocular palsy, dysphagia, slurred speech, and generalized weakness. All required intensive care treatment, and two have had respiratory failure requiring intubation. All patients were treated with Botulism Antitoxin Heptavalent (BAT®) released by the California Department of Public Health (CDPH). The sources of the black tar heroin remain unknown and additional cases may occur. Clusters of botulism cases associated with black tar heroin injection have occurred in Southern California in the past, including five cases in San Diego County in 2010. Background Botulism is a rare and potentially fatal illness caused by the neurotoxin produced by Clostridium botulinum and rarely by other Clostridia species. Routes of exposure vary: patients may present with wound botulism, commonly associated with injection drug use; with foodborne botulism from ingestion of contaminated food items; with infant botulism (the intestinal toxemia form of botulism in patients ≤15months of age); and, rarely, with adult intestinal botulism, or with an iatrogenic exposure to therapeutic botulinum toxin. -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days. -

2 Model Forms, and Other Aids
ANNEX 2 Model Forms, and Other Aids 1. Applicant / Food Employee Interview Form 2. Applicant / Food Employee Reporting Agreement 3. Applicant / Food Employee Medical Referral Form 1 Applicant/Food Employee Interview Form Preventing Transmission of Diseases through Food by Infected Food Employees with Emphasis on illness due to Salmonella Typhi, Shigella spp., Escherichia coli 0157:H7, and Hepatitis A Virus The purpose of this form is to ensure that Applicants to whom a conditional offer of employment has been made and Food Employees advise the Person in Charge of past and current conditions described so that Person in Charge can take appropriate steps to preclude the transmission of foodborne illness. Applicant / Food Employee Name: (print)____________________________________________________________ Address: _______________________________________________________________________________________ _____________________________________________________________________________________________________________ Telephone: Daytime: _________________________ Evening: _________________________ TODAY: Are you now suffering from any of the following: 1. Symptoms Diarrhea? YES / NO Fever? YES / NO Vomiting? YES / NO Jaundice? YES / NO Sore throat with fever? YES / NO 2. Lesions containing pus on the hand, wrist or exposed body part? (such as boils and infected wounds, however small) YES / NO PAST: Have you ever been exposed to or suspected of causing a confirmed outbreak of typhoid fever (Salmonella Typhi), shigellosis (Shigella spp.), Escherichia coli O157:H7 infection (E. coli 0157:H7), or Hepatitis A (hepatitis A virus)? YES / NO If you have, what was the date of the diagnosis?_____________________________ HIGH-RISK CONDITIONS: 1. Have you been exposed to or suspected of causing a confirmed outbreak of typhoid fever, shigellosis, E. coli O157:H7 infection, or hepatitis A? YES / NO 2. Do you live in the same household as a person diagnosed with typhoid fever, shigellosis, hepatitis A, or illness due to E. -

UNIVERSITY of CALIFORNIA Los Angeles Onsite Defluoridation
UNIVERSITY OF CALIFORNIA Los Angeles Onsite Defluoridation Systems for Drinking Water Production A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Civil Engineering by Elaine Ying Ying Wong 2017 © Copyright by Elaine Ying Ying Wong 2017 ABSTRACT OF THE DISSERTATION Onsite Defluoridation Systems for Drinking Water Production by Elaine Ying Ying Wong Doctor of Philosophy in Civil Engineering University of California, Los Angeles, 2017 Professor Michael K. Stenstrom, Chair Fluoride in drinking water has several effects on the teeth and bones. At concentrations of 1-1.5 mg/L, fluoride can strengthen enamel, improving dental health, but at concentrations above 1.5 to 4 mg/L can cause dental fluorosis. At concentrations of 4 -10 mg/L, skeletal fluorosis can occur. There are many areas of the world that have excessive fluoride in drinking water, such as China, India, Sri Lanka, and the Rift Valley countries in Africa. Treatment solutions are needed, especially in poor areas where drinking water treatment plants are not available. On-site or individual treatment alternatives can be attractive if constructed from common materials and if simple enough to be constructed and maintained by users. This dissertation investigates using calcium carbonate as a cost effective sorbent for an onsite defluoridation drinking water system. Batch and column experiments were performed to characterize F- removal properties. Fluoride sorption was described by Freundlich, Langmuir and Dubinin- Radushkevich isotherm models, ii and it was found that the equilibrium time was approximately 3 hours, with approximately 77% of equilibrium concentration reached within 1 hour. -

The Big Five Foodborne Illnesses
The Big Five Foodborne Illnesses: E. Coli Overview: A bacterium that can produce a deadly toxin and causes approximately 73,000 cases of foodborne illness each year in the U.S.; Sources: meat, especially undercooked or raw hamburger, produce and raw milk Incubation Period: 3-4 days Symptoms: Severe diarrhea, cramping, dehydration Prevention: Cook implicated food to 155F; wash hands properly & frequently; correctly wash, rinse & sanitize dishware Shigella Overview: Causes an estimated 300,000 cases of diarrhea illnesses. Poor hygiene causes Shigella to be easily passed from person to person. Sources: salads, milk and dairy products, and unclean water. Incubation Period: 1-7 days Symptoms: Diarrhea, fever, chills, and dehydration Prevention: Wash hands frequently and properly, especially after using the restroom; wash vegetables thoroughly Hepatitis A Overview: Hepatitis A is a liver disease caused by the hepatitis A virus. Hepatitis A can affect anyone. In the United States, hepatitis A can occur in situations ranging from isolated cases of disease to widespread epidemics. Incubation Period: 15-50 days Symptoms: Jaundice, nausea, diarrhea, fever, fatigue, loss of appetite, cramps Prevention: Wash hands frequently and properly, especially after using the restroom Salmonella Overview: Most common cause of foodborne deaths. Responsible for millions of cases of foodborne illness a year; Sources: raw and undercooked eggs, undercooked poultry and meat, dairy products, seafood, fruits and vegetables Incubation Period: 5-72 hours Symptoms: Nausea, vomiting, cramps, and fever Prevention: Cook all food to proper temperatures; chill food rapidly; eliminate sources of cross contamination (i.e. proper meat storage, proper wash, rinse, and sanitize procedure) Norovirus Overview: This virus is the leading cause of diarrhea in the United States. -
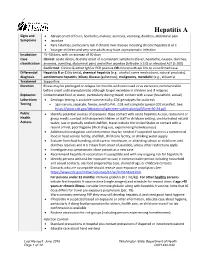
Hepatitis a Reporting Guideline
Hepatitis A Signs and • Abrupt onset of fever, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain Symptoms • Jaundice • Rare fatalities, particularly risk if chronic liver disease including chronic hepatitis B or C • Younger children and very rare adults may have asymptomatic infection Incubation 15–50 days, with an average of 30 days Case Clinical: acute illness, discrete onset of a consistent symptoms (fever, headache, nausea, diarrhea, classification anorexia, vomiting, abdominal pain) and either jaundice (bilirubin ≥ 3.0) or elevated ALT (≥ 200) Confirmed: Clinical & either IgM or PCR positive OR clinical with epi link to a confirmed case Differential Hepatitis B or C (do tests), chemical hepatitis (e.g., alcohol, some medications, natural products), diagnosis autoimmune hepatitis, biliary disease (gallstones), malignancy, metabolic (e.g., Wilson’s) Treatment Supportive Duration Illness may be prolonged or relapse for months with continued virus excretion; communicable before onset until asymptomatic although longer excretion in children and if relapses Exposures Contaminated food or water, particularly during travel; contact with a case (household, sexual) Laboratory • Serologic testing is available commercially; CDC genotypes for outbreak Testing • Spin serum, separate, freeze, send to PHL. CDE will complete special CDC manifest. See: https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf. Public • Identify potential sources of exposure: close contact with acute hepatitis A case, restaurant or Health -

Guidelines for Drinking-Water Quality FIRST ADDENDUM to THIRD EDITION Volume 1 Recommendations WHO Library Cataloguing-In-Publication Data World Health Organization
Guidelines for Drinking-water Quality FIRST ADDENDUM TO THIRD EDITION Volume 1 Recommendations WHO Library Cataloguing-in-Publication Data World Health Organization. Guidelines for drinking-water quality [electronic resource] : incorporating first addendum. Vol. 1, Recommendations. – 3rd ed. Electronic version for the Web. 1.Potable water – standards. 2.Water – standards. 3.Water quality – standards. 4.Guidelines. I. Title. ISBN 92 4 154696 4 (NLM classification: WA 675) © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expres- sion of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Hepatitis a and Hepatitis E Luis S
Hepatitis A and Hepatitis E Luis S. Marsano, MD Professor of Medicine Division of Gastroenterology, Hepatology & Nutrition University of Louisville and Louisville VAMC 2011 Viruses that cause Hepatitis in Humans • Hepatitis A • Lassa virus • Hepatitis B • Rift Valley virus • Hepatitis C • Marburg virus • Hepatitis D • Ebola virus • Hepatitis E • Measles virus • Coxsackie • Human adenovirus • Echovirus • CMV • Yellow fever • EBV • Rubella • HSV • Junin virus (Argentina) • Varicella‐Zoster • Machupo virus (Bolivia) Hepatitis A Hepatitis A • Hepatovirus from Picornavirus family. • 27‐32 nm, linear, (+) sense, simple stranded RNA. • Reservoir: human only • Acquisition: fecal‐oral; concentrated in oysters. Contaminated water or food, men having sex with men, blood during short viremic phase. • Non‐cytopatic; immune mediated injury by lymphocytes Groups at Risk for HAV • Healthy persons who travel to endemic areas, • Workers in occupations with high likelihood of exposure, • Family members of infected patients, • Persons who adopt infants or children from endemic areas, • Men who have sex with men, • Persons who have tested positive for human immunodeficiency virus, • Persons with chronic liver disease, • Persons with clotting factor disorders, • Users of injection and noninjection illicit drugs. Hepatitis A Sero‐Prevalence • Poor sanitation countries: – near 100 % by age 5. • Good sanitation countries (USA) : – 10 % by age 14; – 37 % in adults. Hepatitis A Clinical Features • Incubation: 2‐4 (up to 6) weeks • Children < 2 year: 80% anicteric & asymptomatic • Children > 2 y and adults: 80% icteric & symptomatic • Symptoms: Fatigue (90%), anorexia (85%), jaundice (80%), nausea (75%), low fever (65%), headache, abdominal pain and myalgias. • Duration: usually < 8 weeks Hepatitis A Atypical Manifestations • Relapsing hepatitis: less than 10%; 2 or more bouts of elevated enzymes. -
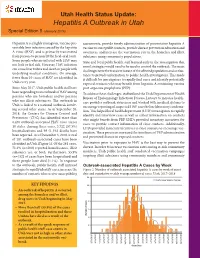
Special Edition Health Status Update: Hepatitis a Outbreak in Utah
Utah Health Status Update: Hepatitis A Outbreak in Utah Special Edition 5 (January 2019) Hepatitis A is a highly contagious, vaccine-pre- assessments, provide timely administration of preventative hepatitis A ventable liver infection caused by the hepatitis vaccine to susceptible contacts, provide disease prevention education and A virus (HAV) and is primarily transmitted awareness, and increase the vaccination rate in the homeless and illicit from person-to-person by the fecal-oral route. substance using community populations. Some people who are infected with HAV may State and local public health staff learned early in the investigation that not look or feel sick. However, HAV infection novel strategies would need to be used to control the outbreak. The main can cause liver failure and death in people with challenges were the transient nature of the affected population and a reluc- underlying medical conditions. On average, tance to provide information to public health investigators. This made fewer than 10 cases of HAV are identified in it difficult for investigators to rapidly find cases and identify potentially Utah every year. exposed contacts who may benefit from hepatitis A containing vaccine Since May 2017, Utah public health staff have post-exposure prophylaxis (PEP). been responding to an outbreak of HAV among To address these challenges, staff utilized the Utah Department of Health persons who are homeless and/or persons Bureau of Epidemiology Infectious Disease Listserv to increase health- who use illicit substances. The outbreak in care provider outbreak awareness and worked with medical systems to Utah is linked to a national outbreak involv- encourage reporting of suspected HAV cases before laboratory confirma- ing several other states. -

Hepatitis E Division of Disease Control What Do I Need to Know?
Division of Disease Control What Do I Need To Know? Hepatitis E What is hepatitis E? Hepatitis E is an infection of the liver caused by the hepatitis E virus. Hepatitis E is not common in North Dakota or in the United States. Who is at risk for hepatitis E? Anyone can get hepatitis E, but those at greater risk include travelers to developing countries, particularly parts of Mexico and countries in South Asia and North Africa. Most outbreaks in developing countries have been associated with contaminated drinking water. Hepatitis E is more common among people ages 15 to 40. Rare cases have occurred in the United States among people with no history of travel to countries where hepatitis E is common. What are the symptoms of hepatitis E? People with hepatitis E may not have symptoms. If symptoms do occur, they are similar to those of other types of viral hepatitis and may include tiredness, loss of appetite, nausea, abdominal discomfort, vomiting, dark urine or jaundice (i.e., yellowing of skin or whites of eyes). The severity of symptoms increases with age. Hepatitis E is more severe among pregnant women, especially in the third trimester. How soon do symptoms appear? Symptoms may appear 15 days to 64 days after exposure, but symptoms usually appear within 26 to 42 days. How is hepatitis E spread? Hepatitis E virus is found in the stools (feces) of infected people. The virus is spread by eating or drinking contaminated food or water. Transmission from person to person occurs less commonly than with hepatitis A virus. -

Hepatitis a Disinfection Guidelines
County of San Diego Department of Environmental Health 5500 Overland Ave. #170 San Diego, CA 92123 858-505-6814 For more information on Hepatitis A or to report individuals with vomiting, diarrhea or fever associated with food consumption, contact: HEPATITIS A Email: [email protected] Phone: (858) 505-6814 DISINFECTION GUIDELINES Website: www.sdcdeh.org Hepatitis A is a liver infection caused by the Hepatitis A virus. Highly contagious, the Hepatitis A virus is usually transmitted by the fecal-oral route, either through person-to-person contact or consumption of contaminated food or water. Contamination can occur when infected persons do not wash their hands properly after going to the bathroom and then touch other objects or food items. Surfaces that are frequently touched should be cleaned and sanitized often. ▪ Toilet Room Surfaces ▪ Kitchen Surfaces ▪ Doorknobs ▪ Recreation Equipment ▪ Light Switch Plates ▪ Phones ▪ Computer Keyboards ▪ Railings ▪ High Chairs ▪ Tables and Chairs ▪ Wheelchairs and Walkers ▪ Remote Controls Effective Disinfection for Steps to Clean Spills of Surfaces Exposed to Hepatitis A Vomit or Feces ▪ Put on personal protective equipment, including two sets of gloves, masks and gowns. ▪ Block-off area immediately. ▪ Clean up visible debris using disposable absorbent ▪ s bleach material (paper towels or other type of disposable cloths) tile and minimize aerosols. floors, ▪ Discard soiled items carefully in an impervious plastic bag. Other ▪ Thoroughly clean affected area To determine if a product is effective against Hepatitis A, ▪ Disinfect area and objects surrounding the review the product label or product specification sheet contamination with an appropriate disinfectant effective and ensure it states that is it effective against Hepatitis A.