Morbidity and Mortality Weekly Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Day 1 Review
Day 1 Review • All food service establishments must have a current and valid permit issued by the New York City Health Department. • Health inspectors have the right to inspect any operating food service or food processing establishment. Inspectors must be given access to all areas of the establishment during an inspection. • According to the New York City Health Code, supervisors of all food service establishments must have a Food Protection Certificate. • Food is any edible substance, ice, beverage or ingredient used or sold for human consumption. • Potentially Hazardous Foods (PHFs) are foods which support rapid growth of microorganisms. • Examples of PHFs include all raw and cooked meats, poultry, milk and milk products, fish, shellfish, tofu, cooked rice, pasta, beans, potatoes and garlic in oil. • The Temperature Danger Zone is between 41°F and 140°F. Within this range, most harmful microorganisms reproduce rapidly. • The three types of thermometers that can be used for measuring food temperatures are: bimetallic stem (range from 0°F to 220°F), thermocouple and thermistor (digital). The use of glass thermometers in a food service establishment is prohibited by law. • Meat inspected by the Unites States Department of Agriculture (USDA) must have a USDA inspection stamp. • Smoked fish must be held at or below 38°F to prevent the growth of the bacteria Clostridium botulinum. • Shellfish must be received with shellfish tags. These tags must be kept on file for at least 90 days after the product is used. • Milk and milk products must either be pasteurized, with sell-by dates of 9 days, or ultra-pasteurized, with sell-by dates of 45 days. -

2016 New Jersey Reportable Communicable Disease Report (January 3, 2016 to December 31, 2016) (Excl
10:34 Friday, June 30, 2017 1 2016 New Jersey Reportable Communicable Disease Report (January 3, 2016 to December 31, 2016) (excl. Sexually Transmitted Diseases, HIV/AIDS and Tuberculosis) (Refer to Technical Notes for Reporting Criteria) Case Jurisdiction Disease Counts STATE TOTAL AMOEBIASIS 98 STATE TOTAL ANTHRAX 0 STATE TOTAL ANTHRAX - CUTANEOUS 0 STATE TOTAL ANTHRAX - INHALATION 0 STATE TOTAL ANTHRAX - INTESTINAL 0 STATE TOTAL ANTHRAX - OROPHARYNGEAL 0 STATE TOTAL BABESIOSIS 174 STATE TOTAL BOTULISM - FOODBORNE 0 STATE TOTAL BOTULISM - INFANT 10 STATE TOTAL BOTULISM - OTHER, UNSPECIFIED 0 STATE TOTAL BOTULISM - WOUND 1 STATE TOTAL BRUCELLOSIS 1 STATE TOTAL CALIFORNIA ENCEPHALITIS(CE) 0 STATE TOTAL CAMPYLOBACTERIOSIS 1907 STATE TOTAL CHIKUNGUNYA 11 STATE TOTAL CHOLERA - O1 0 STATE TOTAL CHOLERA - O139 0 STATE TOTAL CREUTZFELDT-JAKOB DISEASE 4 STATE TOTAL CREUTZFELDT-JAKOB DISEASE - FAMILIAL 0 STATE TOTAL CREUTZFELDT-JAKOB DISEASE - IATROGENIC 0 STATE TOTAL CREUTZFELDT-JAKOB DISEASE - NEW VARIANT 0 STATE TOTAL CREUTZFELDT-JAKOB DISEASE - SPORADIC 2 STATE TOTAL CREUTZFELDT-JAKOB DISEASE - UNKNOWN 1 STATE TOTAL CRYPTOSPORIDIOSIS 198 STATE TOTAL CYCLOSPORIASIS 29 STATE TOTAL DENGUE FEVER - DENGUE 43 STATE TOTAL DENGUE FEVER - DENGUE-LIKE ILLNESS 3 STATE TOTAL DENGUE FEVER - SEVERE DENGUE 4 STATE TOTAL DIPHTHERIA 0 STATE TOTAL EASTERN EQUINE ENCEPHALITIS(EEE) 1 STATE TOTAL EBOLA 0 STATE TOTAL EHRLICHIOSIS/ANAPLASMOSIS - ANAPLASMA PHAGOCYTOPHILUM (PREVIOUSLY HGE) 109 STATE TOTAL EHRLICHIOSIS/ANAPLASMOSIS - EHRLICHIA CHAFFEENSIS (PREVIOUSLY -

Washington State Annual Communicable Disease Report 2008
Washington State COMMUNICABLE DISEASE REPORT 2008 "The Department of Health works to protect and improve the health of people in Washington State." WASHINGTON STATE DEPARTMENT OF HEALTH Epidemiology, Health Statistics and Public Health Laboratories Communicable Disease Epidemiology Section 1610 NE 150th Street Shoreline, WA 98155 206-418-5500 or 1-877-539-4344 COMMUNICABLE DISEASE REPORT 2008 CONTRIBUTORS COMMUNICABLE DISEASE EPIDEMIOLOGY Rebecca Baer, MPH Katelin Bugler, MPH Mary Chadden Erin Chester, MPH Natasha Close, MPH Marisa D’Angeli, MD, MPH Chas DeBolt, RN, MPH Marcia Goldoft, MD, MPH Kathy Lofy, MD Kathryn MacDonald, PhD Nicola Marsden-Haug, MPH Judith May, RN, MPH Tracy Sandifer, MPH Phyllis Shoemaker, BA Deborah Todd, RN, MPH Sherryl Terletter Doreen Terao Wayne Turnberg, PhD, MSPH COMMUNITY AND FAMILY HEALTH Maria Courogen, MPH Kim Field, RN, MSN Salem Gugsa, MPH Tom Jaenicke, MPH, MBA, MES Shana Johnny, RN, MN Julieann Simon, MSPH i Mary Selecky Secretary of Health Maxine Hayes, MD, MPH Health Officer Dennis Dennis, PhD, RN Assistant Secretary Epidemiology, Health Statistics and Public Health Laboratories Judith May, RN, MPH Office Director for Communicable Disease Tony Marfin, MD, MPH, MA State Epidemiologist for Communicable Disease Romesh Gautom, PhD Director, Public Health Laboratories Juliet VanEenwyk, PhD, MS State Epidemiologist for Non-Infectious Disease This report represents Washington State communicable disease surveillance: the ongoing collection, analysis and dissemination of morbidity and mortality data to prevent -

CAHAN San Diego Alert Template
To: CAHAN San Diego Participants Date: April 10, 2018 From: Public Health Services, Epidemiology and Immunizations Services Branch Update: Wound Botulism Cases Associated with Black Tar Heroin This health advisory updates providers about three recently reported wound botulism cases associated with black tar heroin use in San Diego County and provides recommendations on management. Three cases of wound botulism in local heroin users were reported in 2017, two of which were described in a previous CAHAN. Situation In the past month, two confirmed cases and one highly suspect case of wound botulism associated with black tar heroin injection have been reported in San Diego County. The cases are all male, range in age from 28 to 67, and apparently are unknown to each other. They presented with wound infections or abscesses and a recent history of skin or muscle popping black tar heroin. Other symptoms included diplopia, ptosis, extraocular palsy, dysphagia, slurred speech, and generalized weakness. All required intensive care treatment, and two have had respiratory failure requiring intubation. All patients were treated with Botulism Antitoxin Heptavalent (BAT®) released by the California Department of Public Health (CDPH). The sources of the black tar heroin remain unknown and additional cases may occur. Clusters of botulism cases associated with black tar heroin injection have occurred in Southern California in the past, including five cases in San Diego County in 2010. Background Botulism is a rare and potentially fatal illness caused by the neurotoxin produced by Clostridium botulinum and rarely by other Clostridia species. Routes of exposure vary: patients may present with wound botulism, commonly associated with injection drug use; with foodborne botulism from ingestion of contaminated food items; with infant botulism (the intestinal toxemia form of botulism in patients ≤15months of age); and, rarely, with adult intestinal botulism, or with an iatrogenic exposure to therapeutic botulinum toxin. -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Hepatitis a Transmitted by Food
INVITED ARTICLE FOOD SAFETY David Acheson, Section Editor Hepatitis A Transmitted by Food Anthony E. Fiore Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta Hepatitis A is caused by hepatitis A virus (HAV). Transmission occurs by the fecal-oral route, either by direct contact with an HAV-infected person or by ingestion of HAV-contaminated food or water. Foodborne or waterborne hepatitis A outbreaks are relatively uncommon in the United States. However, food handlers with hepatitis A are frequently identified, and evaluation of the need for immunoprophylaxis and implementation of control measures are a considerable burden on public health resources. In addition, HAV-contaminated food may be the source of hepatitis A for an unknown proportion of persons whose source of infection is not identified. FEATURES OF HEPATITIS A and 40%–70% are jaundiced [6]. Children and occasionally young adults can also have inapparent infection, in which Hepatitis A virus (HAV) is classified as a picornavirus. Primates symptoms and elevation of ALT levels are absent but serocon are the only natural host [1]. There is only 1 HAV serotype, version occurs [7]. and immunity after infection is lifelong [2]. After ingestion, Hepatitis A begins with symptoms such as fever, anorexia, uptake in the gastrointestinal tract, and subsequent replication nausea, vomiting, diarrhea, myalgia, and malaise. Jaundice, in the liver, HAV is excreted in bile, and high concentrations dark-colored urine, or light-colored stools might be present at are found in stool specimens. Transmission occurs by the fecal- onset or might follow constitutional symptoms within a few oral route, either by direct contact with an HAV-infected person days. -

2 Model Forms, and Other Aids
ANNEX 2 Model Forms, and Other Aids 1. Applicant / Food Employee Interview Form 2. Applicant / Food Employee Reporting Agreement 3. Applicant / Food Employee Medical Referral Form 1 Applicant/Food Employee Interview Form Preventing Transmission of Diseases through Food by Infected Food Employees with Emphasis on illness due to Salmonella Typhi, Shigella spp., Escherichia coli 0157:H7, and Hepatitis A Virus The purpose of this form is to ensure that Applicants to whom a conditional offer of employment has been made and Food Employees advise the Person in Charge of past and current conditions described so that Person in Charge can take appropriate steps to preclude the transmission of foodborne illness. Applicant / Food Employee Name: (print)____________________________________________________________ Address: _______________________________________________________________________________________ _____________________________________________________________________________________________________________ Telephone: Daytime: _________________________ Evening: _________________________ TODAY: Are you now suffering from any of the following: 1. Symptoms Diarrhea? YES / NO Fever? YES / NO Vomiting? YES / NO Jaundice? YES / NO Sore throat with fever? YES / NO 2. Lesions containing pus on the hand, wrist or exposed body part? (such as boils and infected wounds, however small) YES / NO PAST: Have you ever been exposed to or suspected of causing a confirmed outbreak of typhoid fever (Salmonella Typhi), shigellosis (Shigella spp.), Escherichia coli O157:H7 infection (E. coli 0157:H7), or Hepatitis A (hepatitis A virus)? YES / NO If you have, what was the date of the diagnosis?_____________________________ HIGH-RISK CONDITIONS: 1. Have you been exposed to or suspected of causing a confirmed outbreak of typhoid fever, shigellosis, E. coli O157:H7 infection, or hepatitis A? YES / NO 2. Do you live in the same household as a person diagnosed with typhoid fever, shigellosis, hepatitis A, or illness due to E. -

Reportable Diseases, Emergency Illnesses and Health Conditions, and Reportable Laboratory Findings Changes for 2013
Volume 33, No. 1 January 2013 Reportable Diseases, Emergency Illnesses In this issue... and Health Conditions, and Reportable Reportable Diseases, Emergency Illnesses and Health 1 Conditions, and Reportable Laboratory Findings - Laboratory Findings Changes for 2013 Changes for 2013 List of Reportable Diseases, Emergency Illnesses and 2 Health Conditions - 2013 As required by Connecticut General Statutes Section List of Reportable Laboratory Findings - 2013 3 19a-2a and Section 19a-36-A2 of the Public Health Code, Revisions to the Laboratory Significant Findings 4 the lists of Reportable Diseases, Emergency Illnesses and Report Form (OL-15C) Health Conditions, and Reportable Laboratory Findings Persons Required to Report Reportable Diseases, 4 are revised annually by the Department of Public Health Emergency Illnesses and Health Conditions, and (DPH). An advisory committee, consisting of public Reportable Laboratory Findings health officials, clinicians, and laboratorians, contribute to the process. There are 1 addition, 3 removals, and 1 bovine spongiform encephalopathy that results in vCJD in modification to the healthcare provider list, and 1 people. Free testing of patients for CJD is available at the addition, 1 removal, and 1 modification to the laboratory federally funded National Prion Disease Pathology list for 2013. Surveillance Center, Case Western Reserve University. Changes to the List of Reportable Diseases, Clostridium difficile, community-onset Emergency Illnesses and Health Conditions Reporting of Clostridium difficile, community-onset, Hemolytic uremic syndrome by providers has been removed. Over the past 6 years the Residual serum from hemolytic uremic syndrome incidence of community-onset C. difficile infection (CO (HUS) patients is to be sent to the Katherine A. -

Anasakid Nematodes and Fish
Anasakid Nematodes and Fish Why should we be concerned? Anasakid nematodes are small, round worms. The larvae (immature worms) and adult worms can sometimes can be seen in the organs or flesh of fish, but sometimes not. What can we do to keep safe from these nematodes? It is not known how often there are nematodes in fish in Nunavut. Gut fish as soon as they are killed. If you do not If the larvae in the fish are not killed, gut the fish right after they can cause sickness in humans who harvesting, freeze the fish eat the fish. right away. Cook the fish. Cooking will kill the worms so they can not cause sickness. How does it make If you want to eat the people sick? fish uncooked, freeze it first. Freezing fish will People who eat fish kill both immature and with nematode larvae adult worms. Freeze fish can have tingling in the throat. People for 7 days at -20°C before can also develop stomach ulcers (which eating raw. cause pain in the stomach). -20C˚ 7 days -20˚ It is not known if drying fish kills the nematodes Botulism Why should we be concerned? The botulism bacteria Botulism bacteria can Botulism sickness are found naturally in make toxins if they do not can be very serious in walrus and seal. The have air. The toxins from humans and can even bacteria may also be the bacteria can cause a cause death. found in whale. sickness in humans called “botulism”. How does it make What can we do to keep safe people sick? from botulism? Bacteria can grow and Remove the stomach and make toxins if they do intestines of seal, whale and 4˚ 4˚C or colder not have air. -

UNIVERSITY of CALIFORNIA Los Angeles Onsite Defluoridation
UNIVERSITY OF CALIFORNIA Los Angeles Onsite Defluoridation Systems for Drinking Water Production A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Civil Engineering by Elaine Ying Ying Wong 2017 © Copyright by Elaine Ying Ying Wong 2017 ABSTRACT OF THE DISSERTATION Onsite Defluoridation Systems for Drinking Water Production by Elaine Ying Ying Wong Doctor of Philosophy in Civil Engineering University of California, Los Angeles, 2017 Professor Michael K. Stenstrom, Chair Fluoride in drinking water has several effects on the teeth and bones. At concentrations of 1-1.5 mg/L, fluoride can strengthen enamel, improving dental health, but at concentrations above 1.5 to 4 mg/L can cause dental fluorosis. At concentrations of 4 -10 mg/L, skeletal fluorosis can occur. There are many areas of the world that have excessive fluoride in drinking water, such as China, India, Sri Lanka, and the Rift Valley countries in Africa. Treatment solutions are needed, especially in poor areas where drinking water treatment plants are not available. On-site or individual treatment alternatives can be attractive if constructed from common materials and if simple enough to be constructed and maintained by users. This dissertation investigates using calcium carbonate as a cost effective sorbent for an onsite defluoridation drinking water system. Batch and column experiments were performed to characterize F- removal properties. Fluoride sorption was described by Freundlich, Langmuir and Dubinin- Radushkevich isotherm models, ii and it was found that the equilibrium time was approximately 3 hours, with approximately 77% of equilibrium concentration reached within 1 hour. -

The Big Five Foodborne Illnesses
The Big Five Foodborne Illnesses: E. Coli Overview: A bacterium that can produce a deadly toxin and causes approximately 73,000 cases of foodborne illness each year in the U.S.; Sources: meat, especially undercooked or raw hamburger, produce and raw milk Incubation Period: 3-4 days Symptoms: Severe diarrhea, cramping, dehydration Prevention: Cook implicated food to 155F; wash hands properly & frequently; correctly wash, rinse & sanitize dishware Shigella Overview: Causes an estimated 300,000 cases of diarrhea illnesses. Poor hygiene causes Shigella to be easily passed from person to person. Sources: salads, milk and dairy products, and unclean water. Incubation Period: 1-7 days Symptoms: Diarrhea, fever, chills, and dehydration Prevention: Wash hands frequently and properly, especially after using the restroom; wash vegetables thoroughly Hepatitis A Overview: Hepatitis A is a liver disease caused by the hepatitis A virus. Hepatitis A can affect anyone. In the United States, hepatitis A can occur in situations ranging from isolated cases of disease to widespread epidemics. Incubation Period: 15-50 days Symptoms: Jaundice, nausea, diarrhea, fever, fatigue, loss of appetite, cramps Prevention: Wash hands frequently and properly, especially after using the restroom Salmonella Overview: Most common cause of foodborne deaths. Responsible for millions of cases of foodborne illness a year; Sources: raw and undercooked eggs, undercooked poultry and meat, dairy products, seafood, fruits and vegetables Incubation Period: 5-72 hours Symptoms: Nausea, vomiting, cramps, and fever Prevention: Cook all food to proper temperatures; chill food rapidly; eliminate sources of cross contamination (i.e. proper meat storage, proper wash, rinse, and sanitize procedure) Norovirus Overview: This virus is the leading cause of diarrhea in the United States. -
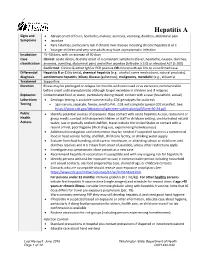
Hepatitis a Reporting Guideline
Hepatitis A Signs and • Abrupt onset of fever, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain Symptoms • Jaundice • Rare fatalities, particularly risk if chronic liver disease including chronic hepatitis B or C • Younger children and very rare adults may have asymptomatic infection Incubation 15–50 days, with an average of 30 days Case Clinical: acute illness, discrete onset of a consistent symptoms (fever, headache, nausea, diarrhea, classification anorexia, vomiting, abdominal pain) and either jaundice (bilirubin ≥ 3.0) or elevated ALT (≥ 200) Confirmed: Clinical & either IgM or PCR positive OR clinical with epi link to a confirmed case Differential Hepatitis B or C (do tests), chemical hepatitis (e.g., alcohol, some medications, natural products), diagnosis autoimmune hepatitis, biliary disease (gallstones), malignancy, metabolic (e.g., Wilson’s) Treatment Supportive Duration Illness may be prolonged or relapse for months with continued virus excretion; communicable before onset until asymptomatic although longer excretion in children and if relapses Exposures Contaminated food or water, particularly during travel; contact with a case (household, sexual) Laboratory • Serologic testing is available commercially; CDC genotypes for outbreak Testing • Spin serum, separate, freeze, send to PHL. CDE will complete special CDC manifest. See: https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf. Public • Identify potential sources of exposure: close contact with acute hepatitis A case, restaurant or Health