Pancreatitis Associated with Viral Hepatitis: Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Pancreatitis Associated with Rotavirus Infection and Review Of
Case Report/Olgu Sunumu İstanbul Med J 2020; 21(1): 78-81 DO I: 10.4274/imj.galenos.2020.88319 Acute Pancreatitis Associated with Rotavirus Infection and Review of The Literature Rotavirüs Enfeksiyonuna Bağlı Akut Pankreatit Olguları ve Literatürün Gözden Geçirilmesi Kamil Şahin, Güzide Doğan University of Health Sciences, Haseki Training and Research Hospital, Department of Pediatrics, İstanbul, Turkey ABSTRACT ÖZ Agents causing acute gastroenteritis are not common causes of Çocuklarda pankreatit etiyolojisinde akut gastroenterit etkenleri pancreatitis etiology in children. Pancreatitis associated with sık görülen sebeplerden değildir. Rotavirüs enfeksiyonuna rotavirus infection is very rare. Cases with acute pancreatitis bağlı görülen pankreatit ise oldukça nadirdir. Rotavirüs during rotavirus gastroenteritis are reported due to rare gastroenteriti sırasında akut pankreatit gelişen olgular, associations. In this article, the causes of acute pancreatitis rotavirüs enfeksiyonuna bağlı akut pankreatitin nadir olması and cases of acute pancreatitis due to rotavirus infection were nedeniyle sunulmuştur. Bu yazıda, akut pankreatit sebepleri ve investigated. Clinical findings were mild, and complications rotavirüse bağlı gelişen akut pankreatit olguları incelenmiştir. were not observed in both of our patients, including a two- İki yaş kız ve üç yaşındaki erkek iki olgumuzda ve literatürde year-old female and a three-year-old male, and other cases değerlendirilen diğer olgularda klinik bulgular hafif seyretmiş, evaluated in the literature. The -

Viral Hepatitis Testing Effective Date: January 1, 2012
Viral Hepatitis Testing Effective Date: January 1, 2012 Scope This guideline provides guidance for the use of laboratory tests to diagnose acute and chronic viral hepatitis in adults (> 19 years) in the primary care setting. General Considerations for Ordering Laboratory Tests Prior to ordering tests for hepatitis, consider the patient’s history, age, risk factors (see below), hepatitis vaccination status, and any available previous hepatitis test results. Risk Factors for Viral Hepatitis include: • Substance use (includes sharing drug snorting, smoking or injection equipment) • High-risk sexual activity or sexual partner with viral hepatitis • Travel to or from high-risk hepatitis endemic areas or exposure during a local outbreak • Immigration from hepatitis B and/or C endemic countries • Household contact with an infected person especially if personal items (e.g., razors, toothbrushes, nail clippers) are shared • Recipient of unscreened blood products* • Needle-stick injury or other occupational exposure (e.g., healthcare workers) • Children born to mothers with chronic hepatitis B or C infection • Attendance at daycare • Contaminated food or water (hepatitis A only) • Tattoos and body piercing • History of incarceration • HIV or other sexually transmitted infection • Hemodialysis *screening of donated blood products for hepatitis C (anti-HCV) began in 1990 in Canada.1 Types of Viral Hepatitis Hepatitis A: causes acute but not chronic hepatitis Hepatitis B: causes acute and chronic hepatitis Hepatitis C: causes chronic hepatitis but rarely manifests as acute hepatitis Hepatitis D: rare and only occurs in patients infected with hepatitis B Hepatitis E: clinically similar to hepatitis A, mostly restricted to endemic areas and occasionally causes chronic infection in immunosuppressed people Others: e.g. -

Chronic Pancreatitis. 2
Module "Fundamentals of diagnostics, treatment and prevention of major diseases of the digestive system" Practical training: "Chronic pancreatitis (CP)" Topicality The incidence of chronic pancreatitis is 4.8 new cases per 100 000 of population per year. Prevalence is 25 to 30 cases per 100 000 of population. Total number of patients with CP increased in the world by 2 times for the last 30 years. In Ukraine, the prevalence of diseases of the pancreas (CP) increased by 10.3%, and the incidence increased by 5.9%. True prevalence rate of CP is difficult to establish, because diagnosis is difficult, especially in initial stages. The average time of CP diagnosis ranges from 30 to 60 months depending on the etiology of the disease. Learning objectives: to teach students to recognize the main symptoms and syndromes of CP; to familiarize students with physical examination methods of CP; to familiarize students with study methods used for the diagnosis of CP, the determination of incretory and excretory pancreatic insufficiency, indications and contraindications for their use, methods of their execution, the diagnostic value of each of them; to teach students to interpret the results of conducted study; to teach students how to recognize and diagnose complications of CP; to teach students how to prescribe treatment for CP. What should a student know? Frequency of CP; Etiological factors of CP; Pathogenesis of CP; Main clinical syndromes of CP, CP classification; General and alarm symptoms of CP; Physical symptoms of CP; Methods of -

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

Treatment Recommendations for Feline Pancreatitis
Treatment recommendations for feline pancreatitis Background is recommended. Fentanyl transdermal patches have become Pancreatitis is an elusive disease in cats and consequently has popular for pain relief because they provide a longer duration of been underdiagnosed. This is owing to several factors. Cats with analgesia. It takes at least 6 hours to achieve adequate fentanyl pancreatitis present with vague signs of illness, including lethargy, levels for pain control; therefore, one recommended protocol is to decreased appetite, dehydration, and weight loss. Physical administer another analgesic, such as intravenous buprenorphine, examination and routine laboratory findings are nonspecific, and at the time the fentanyl patch is placed. The cat is then monitored until recently, there have been limited diagnostic tools available closely to see if additional pain medication is required. Cats with to the practitioner for noninvasively diagnosing pancreatitis. As a chronic pancreatitis may also benefit from pain management, and consequence of the difficulty in diagnosing the disease, therapy options for outpatient treatment include a fentanyl patch, sublingual options are not well understood. buprenorphine, oral butorphanol, or tramadol. Now available, the SNAP® fPL™ and Spec fPL® tests can help rule Antiemetic therapy in or rule out pancreatitis in cats presenting with nonspecific signs Vomiting, a hallmark of pancreatitis in dogs, may be absent or of illness. As our understanding of this disease improves, new intermittent in cats. When present, vomiting should be controlled; specific treatment modalities may emerge. For now, the focus is and if absent, treatment with an antiemetic should still be on managing cats with this disease, and we now have the tools considered to treat nausea. -

Acute Liver Failure J G O’Grady
148 Postgrad Med J: first published as 10.1136/pgmj.2004.026005 on 4 March 2005. Downloaded from REVIEW Acute liver failure J G O’Grady ............................................................................................................................... Postgrad Med J 2005;81:148–154. doi: 10.1136/pgmj.2004.026005 Acute liver failure is a complex multisystemic illness that account for most cases, but a significant number of patients have no definable cause and are evolves quickly after a catastrophic insult to the liver classified as seronegative or of being of indeter- leading to the development of encephalopathy. The minate aetiology. Paracetamol is the commonest underlying aetiology and the pace of progression strongly cause in the UK and USA.2 Idiosyncratic reac- tions comprise another important group. influence the clinical course. The commonest causes are paracetamol, idiosyncratic drug reactions, hepatitis B, and Viral seronegative hepatitis. The optimal care is multidisciplinary ALF is an uncommon complication of viral and up to half of the cases receive liver transplants, with hepatitis, occurring in 0.2%–4% of cases depend- ing on the underlying aetiology.3 The risk is survival rates around 75%–90%. Artificial liver support lowest with hepatitis A, but it increases with the devices remain unproven in efficacy in acute liver failure. age at time of exposure. Hepatitis B can be associated with ALF through a number of ........................................................................... scenarios (table 2). The commonest are de novo infection and spontaneous surges in viral repli- cation, while the incidence of the delta virus cute liver failure (ALF) is a complex infection seems to be decreasing rapidly. multisystemic illness that evolves after a Vaccination should reduce the incidence of Acatastrophic insult to the liver manifesting hepatitis A and B, while antiviral drugs should in the development of a coagulopathy and ameliorate replication of hepatitis B. -

Management of Autoimmune Liver Diseases After Liver Transplantation
Review Management of Autoimmune Liver Diseases after Liver Transplantation Romelia Barba Bernal 1,† , Esli Medina-Morales 1,† , Daniela Goyes 2 , Vilas Patwardhan 1 and Alan Bonder 1,* 1 Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA; [email protected] (R.B.B.); [email protected] (E.M.-M.); [email protected] (V.P.) 2 Department of Medicine, Loyola Medicine—MacNeal Hospital, Berwyn, IL 60402, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-617-632-1070 † These authors contributed equally to this project. Abstract: Autoimmune liver diseases are characterized by immune-mediated inflammation and even- tual destruction of the hepatocytes and the biliary epithelial cells. They can progress to irreversible liver damage requiring liver transplantation. The post-liver transplant goals of treatment include improving the recipient’s survival, preventing liver graft-failure, and decreasing the recurrence of the disease. The keystone in post-liver transplant management for autoimmune liver diseases relies on identifying which would be the most appropriate immunosuppressive maintenance therapy. The combination of a steroid and a calcineurin inhibitor is the current immunosuppressive regimen of choice for autoimmune hepatitis. A gradual withdrawal of glucocorticoids is also recommended. Citation: Barba Bernal, R.; On the other hand, ursodeoxycholic acid should be initiated soon after liver transplant to prevent Medina-Morales, E.; Goyes, D.; recurrence and improve graft and patient survival in primary biliary cholangitis recipients. Unlike the Patwardhan, V.; Bonder, A. Management of Autoimmune Liver previously mentioned autoimmune diseases, there are not immunosuppressive or disease-modifying Diseases after Liver Transplantation. -
Pancreatic Disorders in Inflammatory Bowel Disease
Submit a Manuscript: http://www.wjgnet.com/esps/ World J Gastrointest Pathophysiol 2016 August 15; 7(3): 276-282 Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx ISSN 2150-5330 (online) DOI: 10.4291/wjgp.v7.i3.276 © 2016 Baishideng Publishing Group Inc. All rights reserved. MINIREVIEWS Pancreatic disorders in inflammatory bowel disease Filippo Antonini, Raffaele Pezzilli, Lucia Angelelli, Giampiero Macarri Filippo Antonini, Giampiero Macarri, Department of Gastro- acute pancreatitis or chronic pancreatitis has been rec enterology, A.Murri Hospital, Polytechnic University of Marche, orded in patients with inflammatory bowel disease (IBD) 63900 Fermo, Italy compared to the general population. Although most of the pancreatitis in patients with IBD seem to be related to Raffaele Pezzilli, Department of Digestive Diseases and Internal biliary lithiasis or drug induced, in some cases pancreatitis Medicine, Sant’Orsola-Malpighi Hospital, 40138 Bologna, Italy were defined as idiopathic, suggesting a direct pancreatic Lucia Angelelli, Medical Oncology, Mazzoni Hospital, 63100 damage in IBD. Pancreatitis and IBD may have similar Ascoli Piceno, Italy presentation therefore a pancreatic disease could not be recognized in patients with Crohn’s disease and ulcerative Author contributions: Antonini F designed the research; Antonini colitis. This review will discuss the most common F and Pezzilli R did the data collection and analyzed the data; pancreatic diseases seen in patients with IBD. Antonini F, Pezzilli R and Angelelli L wrote the paper; Macarri G revised the paper and granted the final approval. Key words: Pancreas; Pancreatitis; Extraintestinal mani festations; Exocrine pancreatic insufficiency; Ulcerative Conflictofinterest statement: The authors declare no conflict colitis; Crohn’s disease; Inflammatory bowel disease of interest. -

Necrotizing Pancreatitis and Gas Forming Organisms
JOP. J Pancreas (Online) 2016 Nov 08; 17(6):649-652. CASE REPORT Necrotizing Pancreatitis and Gas Forming Organisms Theadore Hufford, Terrence Lerner Metropolitan Group Hospitals Residency in General Surgery, University of Illinois, United States ABSTRACT Context Acute Pancreatitis is a common disease of the gastrointestinal tract that accounts for thousands of hospital admissions in the United States every year. Severe acute necrotic pancreatitis has a high mortality rate if left untreated, and always requires surgical intervention. The timing of surgical intervention is of importance. Here we present a case of a patient with severe necrotizing pancreatitis with possible gas producing bacteria in the retroperitoneum shown on imaging and cultures. Case Report The patient is a Seventy- two-year old male presenting to the emergency department with complaining of severe epigastric pain for the past 48 hours. The labs and clinical symptoms were consistent with pancreatitis. However, the imaging showed necrotic pancreatitis that required immediate intervention. During the course of six weeks, the patient underwent numerous surgical procedures to debride the necrotic pancreas. The patient was ultimately clinically stable to be discharged and transferred to a skilled-nursing facility, but returned 3 days later with a post- surgical wound infection vs. Conclusion The patient ultimately expired 7 days after his second admission to the hospital due to multi-organ failure secondary to sepsis. anastomotic leak with enterocutaneous fistula. INTRODUCTION has proven to decrease mortality by about 40% in most cases [6]. Finding the cause of the infection is of the Acute Pancreatitis (AP) is a common disease of the utmost importance in necrotizing pancreatitis cases. -
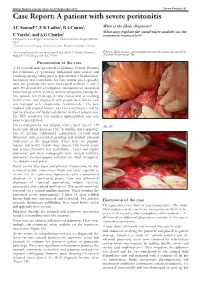
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Spontaneous Bacterial Peritonitis: Recent Guidelines and Beyond R Wiest,1 a Krag,2 a Gerbes3
Downloaded from gut.bmj.com on May 31, 2012 - Published by group.bmj.com Recent advances in clinical practice Spontaneous bacterial peritonitis: recent guidelines and beyond R Wiest,1 A Krag,2 A Gerbes3 1Department for visceral surgery INTRODUCTION prognosis in various cohorts of patients with SBP and medicine, University Spontaneous bacterial peritonitis (SBP) is the most are summarised in figure 1 and include age,16 20 Hospital Bern, Switzerland 18 20 23 16 18 2 frequent and life-threatening infection in patients Child score, intensive care, nosocomial Department of 18 24 25 Gastroenterology, Hvidovre with liver cirrhosis requiring prompt recognition origin, hepatic encephalopathy, elevated 26 University Hospital, and treatment. It is defined by the presence of >250 serum creatinine and bilirubin, lack of infection Copenhagen, Denmark polymorphonuclear cells (PMN)/mm3 in ascites in resolution/need to escalate treatment and culture 3 e Klinikum of the University of the absence of an intra-abdominal source of infec- positivity27 29 as well as the presence of bacter- Munich, Munich, Germany tion or malignancy. In this review we discuss the aemia30 and CARD15/NOD2 variants as a genetic fl 31 Correspondence to current opinions re ected by recent guidelines risk factor. It is important to stress in this context Professor Dr Reiner Wiest, (American Association for the Study of Liver that the only factors that are modifiable in this Department for visceral surgery Diseases, European Association for the Study of the scenario are timely diagnosis and effective first-line and medicine, University Liver, Deutsche Gesellschaft für Verdauungs- und treatment. Hospital Bern, 3010 Bern, 1e4 Switzerland; Stoffwechselkrankheiten), with particular focus [email protected] on controversial issues as well as open questions Bacterial translocation (BT) and pathophysiology that need to be addressed in the future. -

Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV.