Prevalence of Rotavirus, Adenovirus, Hepatitis a Virus and Enterovirus in Water Samples Collected from Different Region of Peshawar, Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Positive Rhinovirus/Enterovirus Detection and SARS-Cov-2 Persistence Beyond the Acute Infection Phase: an Intra-Household Surveillance Study
viruses Communication The Positive Rhinovirus/Enterovirus Detection and SARS-CoV-2 Persistence beyond the Acute Infection Phase: An Intra-Household Surveillance Study Pedro Brotons 1,2,3, Iolanda Jordan 1,3,4, Quique Bassat 3,5,6,7,8 , Desiree Henares 1,3, Mariona Fernandez de Sevilla 1,3,5, Sara Ajanovic 7, Alba Redin 1,2, Vicky Fumado 1,5, Barbara Baro 7 , Joana Claverol 9, Rosauro Varo 7 , Daniel Cuadras 9 , Jochen Hecht 10, Irene Barrabeig 3,11, Juan Jose Garcia-Garcia 1,3,5, Cristian Launes 1,3,5,† and Carmen Muñoz-Almagro 1,2,3,12,*,† 1 Pediatric Infectious Diseases Research Group, Institut de Recerca Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain; [email protected] (P.B.); [email protected] (I.J.); [email protected] (D.H.); [email protected] (M.F.d.S.); [email protected] (A.R.); [email protected] (V.F.); [email protected] (J.J.G.-G.); [email protected] (C.L.) 2 Department of Medicine, School of Medicine, Universitat Internacional de Catalunya, Sant Cugat, 08195 Barcelona, Spain 3 Consorcio de Investigacion Biomédica en Red Epidemiologia y Salud Pública (CIBERESP), 28029 Madrid, Spain; [email protected] (Q.B.); [email protected] (I.B.) 4 Pediatric Intensive Care Unit, Hospital Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain 5 Pediatrics Department, Hospital Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain 6 Centro de Investigação em Saúde de Manhiça (CISM), Manhiça 1929, Mozambique Citation: Brotons, P.; Jordan, I.; 7 ISGlobal, Hospital Clínic-Universitat de Barcelona, 08036 Barcelona, Spain; [email protected] (S.A.); Bassat, Q.; Henares, D.; Fernandez de [email protected] (B.B.); [email protected] (R.V.) Sevilla, M.; Ajanovic, S.; Redin, A.; 8 Institució Catalana de Recerca i Estudis Avançats (ICREA), 08010 Barcelona, Spain Fumado, V.; Baro, B.; Claverol, J.; et al. -

Viral Hepatitis Testing Effective Date: January 1, 2012
Viral Hepatitis Testing Effective Date: January 1, 2012 Scope This guideline provides guidance for the use of laboratory tests to diagnose acute and chronic viral hepatitis in adults (> 19 years) in the primary care setting. General Considerations for Ordering Laboratory Tests Prior to ordering tests for hepatitis, consider the patient’s history, age, risk factors (see below), hepatitis vaccination status, and any available previous hepatitis test results. Risk Factors for Viral Hepatitis include: • Substance use (includes sharing drug snorting, smoking or injection equipment) • High-risk sexual activity or sexual partner with viral hepatitis • Travel to or from high-risk hepatitis endemic areas or exposure during a local outbreak • Immigration from hepatitis B and/or C endemic countries • Household contact with an infected person especially if personal items (e.g., razors, toothbrushes, nail clippers) are shared • Recipient of unscreened blood products* • Needle-stick injury or other occupational exposure (e.g., healthcare workers) • Children born to mothers with chronic hepatitis B or C infection • Attendance at daycare • Contaminated food or water (hepatitis A only) • Tattoos and body piercing • History of incarceration • HIV or other sexually transmitted infection • Hemodialysis *screening of donated blood products for hepatitis C (anti-HCV) began in 1990 in Canada.1 Types of Viral Hepatitis Hepatitis A: causes acute but not chronic hepatitis Hepatitis B: causes acute and chronic hepatitis Hepatitis C: causes chronic hepatitis but rarely manifests as acute hepatitis Hepatitis D: rare and only occurs in patients infected with hepatitis B Hepatitis E: clinically similar to hepatitis A, mostly restricted to endemic areas and occasionally causes chronic infection in immunosuppressed people Others: e.g. -

Hesperetin Protects Crayfish Procambarus Clarkii Against White
Fish and Shellfish Immunology 93 (2019) 116–123 Contents lists available at ScienceDirect Fish and Shellfish Immunology journal homepage: www.elsevier.com/locate/fsi Full length article Hesperetin protects crayfish Procambarus clarkii against white spot syndrome virus infection T Xiyi Qian, Fei Zhu* Zhejiang Provincial Engineering Laboratory for Animal Health Inspection and Internet Technology, College of Animal Science and Technology, Zhejiang Agriculture and Forestry University, Hangzhou, 311300, China ARTICLE INFO ABSTRACT Keywords: Hesperetin is a natural flavanone compound, which mainly exists in lemons and oranges, and has potential Hesperetin antiviral and anticancer activities. In this study, hesperetin was used in a crayfish pathogen challenge to discover WSSV its effects on the innate immune system of invertebrates. The crayfish Procambarus clarkii was used as an ex- Innate immunity perimental model and challenged with white spot syndrome virus (WSSV). Pathogen challenge experiments Procambarus clarkii showed that hesperetin treatment significantly reduced the mortality caused by WSSV infection, while the VP28 copies of WSSV were also reduced. Quantitative reverse transcriptase polymerase chain reaction revealed that hesperetin increased the expression of several innate immune-related genes, including NF-kappaB and C-type lectin. Further analysis showed that hesperetin treatment plays a positive effects on three immune parameters like total hemocyte count, phenoloxidase and superoxide dismutase activity. Nevertheless, whether or not in- fected with WSSV, hesperetin treatment would significantly increase the hemocyte apoptosis rates in crayfish. These results indicated that hesperetin could regulate the innate immunity of crayfish, and delaying and re- ducing the mortality after WSSV challenge. Therefore, the present study provided novel insights into the po- tential therapeutic or preventive functions associated with hesperetin to regulate crayfish immunity and protect crayfish against WSSV infection, provide certain theoretical basis for production practice. -

Prevention & Control of Viral Hepatitis Infection
Prevention & Control of Viral Hepatitis Infection: A Strategy for Global Action © World Health Organization 2011. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. Table of contents Disease burden 02 What is viral hepatitis? 05 Prevention & control: a tailored approach 06 Global Achievements 08 Remaining challenges 10 World Health Assembly: a mandate for comprehensive prevention & control 13 WHO goals and strategy -

Phage Display-Derived Cross-Reactive Neutralizing Antibody Against Enterovirus 71 and Coxsackievirus A16
Jpn. J. Infect. Dis., 69, 66–74, 2016 Original Article Phage Display-Derived Cross-Reactive Neutralizing Antibody against Enterovirus 71 and Coxsackievirus A16 Xiao Zhang1†, Chunyun Sun2†, Xiangqian Xiao1,LinPang3, Sisi Shen1, Jie Zhang2, Shan Cen4,BurtonB.Yang5, Yuming Huang3, Wang Sheng1*, and Yi Zeng1 1College of Life Science and Bioengineering, Beijing University of Technology, Beijing; 2Sinocelltech, Cell Engineering Center, Chinese Academy of Medical Science, Beijing; 3Beijing Ditan Hospital, Capital Medical University, Beijing; 4Department of Virology, Institute of Medicinal Biotechnology, Chinese Academy of Medical Science, Beijing, China; and 5Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada SUMMARY: Enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) are members of the Picornaviri- dae family and are considered the main causative agents of hand, foot and mouth disease (HFMD). In recent decades large HFMD outbreaks caused by EV71 and CVA16 have become significant public health concerns in the Asia-Pacific region. Vaccines and antiviral drugs are unavailable to prevent EV71 and CVA16 infection. In the current study, a chimeric antibody targeting a highly conserved peptide in the EV71 VP4 protein was isolated by using a phage display technique. The antibody showed cross- neutralizing capability against EV71 and CVA16 in vitro. The results suggest that this phage display- derived antibody will have great potential as a broad neutralizing antibody against EV71 and CVA16 after affinity maturation and humanization. potential for new viral recombinants of EV71 and INTRODUCTION CVA16 to emerge have been documented (13–15). These Enterovirus 71 (EV71) and coxsakievirus A16 findings suggest that both EV71 and CVA16 should be (CVA16) are non-enveloped RNA viruses of the targeted for vaccine and therapeutic development for ef- Picornaviridae family. -

NSP4)-Induced Intrinsic Apoptosis
viruses Article Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis Rakesh Sarkar †, Satabdi Nandi †, Mahadeb Lo, Animesh Gope and Mamta Chawla-Sarkar * Division of Virology, National Institute of Cholera and Enteric Diseases, P-33, C.I.T. Road Scheme-XM, Beliaghata, Kolkata 700010, India; [email protected] (R.S.); [email protected] (S.N.); [email protected] (M.L.); [email protected] (A.G.) * Correspondence: [email protected]; Tel.: +91-33-2353-7470; Fax: +91-33-2370-5066 † These authors contributed equally to this work. Abstract: Viral infections lead to expeditious activation of the host’s innate immune responses, most importantly the interferon (IFN) response, which manifests a network of interferon-stimulated genes (ISGs) that constrain escalating virus replication by fashioning an ill-disposed environment. Interestingly, most viruses, including rotavirus, have evolved numerous strategies to evade or subvert host immune responses to establish successful infection. Several studies have documented the induction of ISGs during rotavirus infection. In this study, we evaluated the induction and antiviral potential of viperin, an ISG, during rotavirus infection. We observed that rotavirus infection, in a stain independent manner, resulted in progressive upregulation of viperin at increasing time points post-infection. Knockdown of viperin had no significant consequence on the production of total Citation: Sarkar, R.; Nandi, S.; Lo, infectious virus particles. Interestingly, substantial escalation in progeny virus release was observed M.; Gope, A.; Chawla-Sarkar, M. upon viperin knockdown, suggesting the antagonistic role of viperin in rotavirus release. Subsequent Viperin, an IFN-Stimulated Protein, studies unveiled that RV-NSP4 triggered relocalization of viperin from the ER, the normal residence Delays Rotavirus Release by Inhibiting of viperin, to mitochondria during infection. -
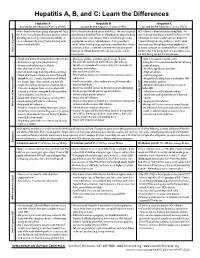
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Rotavirus Fact Sheet
ROTAVIRUS FACT SHEET What is Rotavirus? commonly spreads in families, hospitals, and childcare Rotavirus is a virus that commonly causes inflammation centers. A person with rotavirus disease is most likely to of the stomach and intestines (acute gastroenteritis). spread the virus from the time they are sick through the first 3 days after they recover. Who can get Rotavirus disease? Anyone. However, it is most common in infants and Is there a vaccine for Rotavirus? young children. A person may be infected more than Yes. The vaccine is highly effective at preventing severe once. Nearly every child who is not vaccinated as an rotavirus disease in infants and young children. The infant is expected to be infected within the first years of vaccine is given orally starting at age 2 months as a life. series of 2 doses or 3 doses, depending on the specific type. What are the symptoms of Rotavirus disease? Children with rotavirus disease may have severe watery How can the spread of Rotavirus be prevented? diarrhea, vomiting, fever, abdominal pain, and loss of The virus spreads so easily that frequent handwashing appetite. Vomiting and diarrhea can lead to dehydration. with soap and water is important, but not sufficient for Signs of dehydration include decreased urination, dry controlling the spread of the disease. Rotavirus mouth and throat, and feeling dizzy when standing up. A vaccination is the best way to protect children against child who is dehydrated may have few or no tears when rotavirus disease. crying and be unusually sleepy or fussy. Usually a person’s first infection with rotavirus tends to cause the How is Rotavirus disease treated? most severe symptoms. -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Detailed Review Paper on Rotavirus Vaccines
Rotavirus Vaccines 17 March 2009 Detailed Review Paper on Rotavirus Vaccines To be presented to the WHO Strategic Advisory Group of Experts (SAGE) on Immunization, April 2009 Ad-hoc group of experts on rotavirus vaccines Chair : G. Peter Members: T. Aguado, Z. Bhutta, L. De Oliveira, K. Neuzil, U. Parashar, D. Steele WHO Secretariat: C. Mantel, S. Wang, G. Mayers, E. Derobert Rapporteur: D. Payne 1 Rotavirus Vaccines 17 March 2009 Table of Contents I. Rotavirus Epidemiology and Rationale for Vaccination 1. Disease burden 2. Rationale for vaccination as the primary preventive measure II. Rotavirus Vaccine Efficacy and Safety in Pivotal Pre-Licensure Trials Brief summary of rotavirus vaccines 1. Rotarix ® 2. RotaTeq ® III. Newly Available Data from Clinical Trials in Africa and Asia and Post-introduction Vaccine Effectiveness Evaluations in the Americas 1. South Africa and Malawi clinical trials (Rotarix ®) 2. Hong Kong, Taiwan, and Singapore clinical trials (Rotarix ®) 3. Nicaragua post-introduction vaccine effectiveness case- control study (RotaTeq ®) 4. El Salvador post-introduction vaccine effectiveness case- control study (Rotarix ®) 5. United States post-licensure impact evaluation studies 6. Status of other ongoing studies IV. Vaccine Safety, Co-Administration, and Special Populations 1. Vaccine safety 2. Co-administration with other vaccines, particularly OPV 3. HIV-infected populations 4. Breast-feeding and Pre-term Infants V. Vaccine Schedules and Age Restrictions VI. Vaccine Cost-effectiveness and Decision-Making Regarding Program Implementation 1. Cost-effectiveness and affordability 2. Decision-making regarding vaccine introduction VII. Vaccine Program Implementation and Vaccine Delivery Logistics VIII. Integration with Diarrheal Control and Other Health Interventions and Communication 1. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

(Hadv), Human Enterovirus (Hev), and Genogroup a Rotavirus (GARV) in Tap Water in Southern Brazil M
526 © IWA Publishing 2014 Journal of Water and Health | 12.3 | 2014 Human adenovirus (HAdV), human enterovirus (hEV), and genogroup A rotavirus (GARV) in tap water in southern Brazil M. Kluge, J. D. Fleck, M. C. Soliman, R. B. Luz, R. B. Fabres, J. Comerlato, J. V. S. Silva, R. Staggemeier, A. D. Vecchia, R. Capalonga, A. B. Oliveira, A. Henzel, C. Rigotto and F. R. Spilki ABSTRACT The effects of viral gastroenteritis are more devastating in children than in any other age category. M. Kluge J. D. Fleck Thus, children exposed to the consumption of low quality water are at an increased risk of infection, M. C. Soliman R. B. Luz especially in regions where sanitation is inadequate. The present study aimed to provide a survey of R. B. Fabres J. V. S. Silva the occurrence of representative enteric viruses: human adenovirus (HAdV), human enteroviruses R. Staggemeier (hEV), and genogroup A rotavirus (GARV) in tap water samples collected in public schools located at A. D. Vecchia A. Henzel six municipalities of Rio Grande do Sul, southern Brazil. Seventy-three schools were included in the C. Rigotto F. R. Spilki (corresponding author) study and tap water samples were analyzed by conventional PCR for the presence of HAdV, hEV, and Laboratório de Microbiologia Molecular (LMM), Instituto de Ciências da Saúde (ICS), GARV genomes. hEV showed the highest detection rate (27.4%), followed by HAdV (23.3%), and GARV Universidade Feevale, Novo Hamburgo, RS, (16.4%). New approaches to water monitoring should be considered to promote a better water Brazil E-mail: [email protected] quality and reduce the risk of waterborne diseases, especially considering drinking water to be J.