HUMAN ADENOVIRUS Credibility of Association with Recreational Water: Strongly Associated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
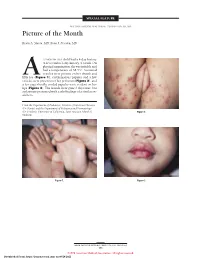
Picture of the Month
SPECIAL FEATURE SECTION EDITOR: WALTER W. TUNNESSEN, JR, MD Picture of the Month Kevin A. Slavin, MD; Ilona J. Frieden, MD 15-MONTH-OLD child had a 4-day history of fever and a 1-day history of a rash. On physical examination she was irritable and had a temperature of 38.3°C. Scattered vesicles were present on her thumb and Afifth toe (Figure 1), erythematous papules and a few vesicles were present over her perineum (Figure 2), and a few superficially eroded papules were evident on her lips (Figure 3). The lesions were gone 3 days later, but a playmate presented with early findings of a similar ex- anthem. From the Department of Pediatrics, Division of Infectious Diseases (Dr Slavin) and the Department of Pediatrics and Dermatology (Dr Frieden), University of California, San Francisco School of Figure 2. Medicine. Figure 1. Figure 3. ARCH PEDIATR ADOLESC MED/ VOL 152, MAY 1998 505 ©1998 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/28/2021 Denouement and Discussion Hand-Foot-and-Mouth Disease Figure 1. Vesicles are present on the thumb and fifth toe. foot-and-mouth disease).1,2,5-9 The lesions on the but- tocks are of the same size and typical of the early forms Figure 2. Multiple erythematous papules and a few scattered vesicles are of the exanthem, but they are not frequently vesicular present over the perineum. in nature. Lesions involving the perineum seem to be more Figure 3. Superficially eroded papules are present on the lips. common in children who wear diapers, suggesting that friction or minor trauma may play a role in the develop- ment of lesions. -

Nasoswab ® Brochure
® NasoSwab One Vial... Multiple Pathogens Simple & Convenient Nasal Specimen Collection Medical Diagnostic Laboratories, L.L.C. 2439 Kuser Road • Hamilton, NJ 08690-3303 www.mdlab.com • Toll Free 877 269 0090 ® NasoSwab MULTIPLE PATHOGENS The introduction of molecular techniques, such as the Polymerase Chain Reaction (PCR) method, in combination with flocked swab technology, offers a superior route of pathogen detection with a high diagnostic specificity and sensitivity. MDL offers a number of assays for the detection of multiple pathogens associated with respiratory tract infections. The unrivaled sensitivity and specificity of the Real-Time PCR method in detecting infectious agents provides the clinician with an accurate and rapid means of diagnosis. This valuable diagnostic tool will assist the clinician with diagnosis, early detection, patient stratification, drug prescription, and prognosis. Tests currently available utilizing ® the NasoSwab specimen collection platform are listed below. • One vial, multiple pathogens Acinetobacter baumanii • DNA amplification via PCR technology Adenovirus • Microbial drug resistance profiling Bordetella parapertussis • High precision robotic accuracy • High diagnostic sensitivity & specificity Bordetella pertussis (Reflex to Bordetella • Specimen viability up to 5 days after holmesii by Real-Time PCR) collection Chlamydophila pneumoniae • Test additions available up to 30 days Coxsackie virus A & B after collection • No refrigeration or freezing required Enterovirus D68 before or after collection -

ADENOVIRUS Adenoviruses Are Common Viruses That Typically Cause Mild Cold- Or Flu-Like Illness
ADENOVIRUS Adenoviruses are common viruses that typically cause mild cold- or flu-like illness. Adenoviruses can cause illness in people of all ages any time of year. ADENOVIRUS Adenoviruses can cause a wide range of illnesses including • common cold- or flu-like symptoms SYMPTOMS • fever • sore throat • pink eye (conjunctivitis) • acute bronchitis (inflammation of the airways of the lungs, sometimes called a “chest cold”) • pneumonia (infection of the lungs, occasionally severe) • diarrhea • acute gastroenteritis (inflammation of the stomach or intestines causing diarrhea, vomiting, nausea, and stomach pain) Less common illnesses caused by adenovirus include bladder infection or inflammation and neurologic disease (conditions that affect the brain and spinal cord). HOW Adenoviruses are usually spread from an infected person to others through ADENOVIRUSES • close personal contact, such as touching or shaking hands SPREAD • the air by coughing and sneezing • touching an object or surface with adenoviruses on it, then touching your mouth, nose, or eyes before washing your hands • contact with stool, for example, during diaper changing Adenoviruses are often resistant to common disinfectants and can remain infectious for long periods of time on surfaces and objects. WHO IS AT RISK FOR SEVERE ADENOVIRUS INFECTION? People with weakened immune systems (including from medications they are taking or from heart or lung diseases) are at higher risk for developing severe adenovirus infection. Certain types of this virus have been linked to more severe illness. Rarely, otherwise healthy people with adenovirus infections will become so ill that they need to be hospitalized and may die. Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases HOW TO Protect yourself from getting sick. -

The Positive Rhinovirus/Enterovirus Detection and SARS-Cov-2 Persistence Beyond the Acute Infection Phase: an Intra-Household Surveillance Study
viruses Communication The Positive Rhinovirus/Enterovirus Detection and SARS-CoV-2 Persistence beyond the Acute Infection Phase: An Intra-Household Surveillance Study Pedro Brotons 1,2,3, Iolanda Jordan 1,3,4, Quique Bassat 3,5,6,7,8 , Desiree Henares 1,3, Mariona Fernandez de Sevilla 1,3,5, Sara Ajanovic 7, Alba Redin 1,2, Vicky Fumado 1,5, Barbara Baro 7 , Joana Claverol 9, Rosauro Varo 7 , Daniel Cuadras 9 , Jochen Hecht 10, Irene Barrabeig 3,11, Juan Jose Garcia-Garcia 1,3,5, Cristian Launes 1,3,5,† and Carmen Muñoz-Almagro 1,2,3,12,*,† 1 Pediatric Infectious Diseases Research Group, Institut de Recerca Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain; [email protected] (P.B.); [email protected] (I.J.); [email protected] (D.H.); [email protected] (M.F.d.S.); [email protected] (A.R.); [email protected] (V.F.); [email protected] (J.J.G.-G.); [email protected] (C.L.) 2 Department of Medicine, School of Medicine, Universitat Internacional de Catalunya, Sant Cugat, 08195 Barcelona, Spain 3 Consorcio de Investigacion Biomédica en Red Epidemiologia y Salud Pública (CIBERESP), 28029 Madrid, Spain; [email protected] (Q.B.); [email protected] (I.B.) 4 Pediatric Intensive Care Unit, Hospital Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain 5 Pediatrics Department, Hospital Sant Joan de Déu, Esplugues de Llobregat, 08950 Barcelona, Spain 6 Centro de Investigação em Saúde de Manhiça (CISM), Manhiça 1929, Mozambique Citation: Brotons, P.; Jordan, I.; 7 ISGlobal, Hospital Clínic-Universitat de Barcelona, 08036 Barcelona, Spain; [email protected] (S.A.); Bassat, Q.; Henares, D.; Fernandez de [email protected] (B.B.); [email protected] (R.V.) Sevilla, M.; Ajanovic, S.; Redin, A.; 8 Institució Catalana de Recerca i Estudis Avançats (ICREA), 08010 Barcelona, Spain Fumado, V.; Baro, B.; Claverol, J.; et al. -

Trunkloads of Viruses
COMMENTARY Trunkloads of Viruses Philip E. Pellett Department of Immunology and Microbiology, Wayne State University School of Medicine, Detroit, Michigan, USA Elephant populations are under intense pressure internationally from habitat destruction and poaching for ivory and meat. They also face pressure from infectious agents, including elephant endotheliotropic herpesvirus 1 (EEHV1), which kills ϳ20% of Asian elephants (Elephas maximus) born in zoos and causes disease in the wild. EEHV1 is one of at least six distinct EEHV in a phylogenetic lineage that appears to represent an ancient but newly recognized subfamily (the Deltaherpesvirinae) in the family Herpesviridae. lephant endotheliotropic herpesvirus 1 (EEHV1) causes a rap- the Herpesviridae (the current complete list of approved virus tax- Downloaded from Eidly progressing and usually fatal hemorrhagic disease that ons is available at http://ictvonline.org/). In addition, approxi- occurs in the wild in Asia and affects ϳ20% of Asian elephant mately 200 additional viruses detected using methods such as (Elephas maximus) calves born in zoos in the United States and those described above await formal consideration (V. Lacoste, Europe (1). About 60% of juvenile deaths of captive elephants are personal communication). With very few exceptions, the amino attributed to such infections. Development of control measures acid sequence of a small conserved segment of the viral DNA poly- has been hampered by the lack of systems for culture of the virus in merase (ϳ150 amino acids) is sufficient to not only reliably iden- laboratories. Its genetic study has been restricted to analysis of tify a virus as belonging to the evolutionary lineage represented by blood, trunk wash fluid, and tissue samples collected during nec- the Herpesviridae, but also their subfamily, and in most cases a http://jvi.asm.org/ ropsies. -

Guide for Common Viral Diseases of Animals in Louisiana
Sampling and Testing Guide for Common Viral Diseases of Animals in Louisiana Please click on the species of interest: Cattle Deer and Small Ruminants The Louisiana Animal Swine Disease Diagnostic Horses Laboratory Dogs A service unit of the LSU School of Veterinary Medicine Adapted from Murphy, F.A., et al, Veterinary Virology, 3rd ed. Cats Academic Press, 1999. Compiled by Rob Poston Multi-species: Rabiesvirus DCN LADDL Guide for Common Viral Diseases v. B2 1 Cattle Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 2 Deer and Small Ruminants Please click on the principle system involvement Generalized viral disease Respiratory viral disease Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 3 Swine Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 4 Horses Please click on the principle system involvement Generalized viral diseases Neurological viral diseases Respiratory viral diseases Enteric viral diseases Abortifacient/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 5 Dogs Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. -

Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet
Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet Hand, foot, and mouth disease is caused by one of several types of viruses Hand, foot, and mouth disease is usually characterized by tiny blisters on the inside of the mouth and the palms of the hands, fingers, soles of the feet. It is commonly caused by coxsackievirus A16 (an enterovirus), and less often by other types of viruses. Anyone can get hand, foot, and mouth disease Young children are primarily affected, but it may be seen in adults. Most cases occur in the summer and early fall. Outbreaks may occur among groups of children especially in child care centers or nursery schools. Symptoms usually appear 3 to 5 days after exposure. Hand, foot, and mouth disease is usually spread through person-to-person contact People can spread the disease when they are shedding the virus in their feces. It is also spread by the respiratory tract from mouth or respiratory secretions (such as from saliva on hands or toys). The virus has also been found in the fluid from the skin blisters. The infection is spread most easily during the acute phase/stage of illness when people are feeling ill, but the virus can be spread for several weeks after the onset of infection. The symptoms are much like a common cold with a rash The rash appears as blisters or ulcers in the mouth, on the inner cheeks, gums, sides of the tongue, and as bumps or blisters on the hands, feet, and sometimes other parts of the skin. The skin rash may last for 7 to 10 days. -

Hesperetin Protects Crayfish Procambarus Clarkii Against White
Fish and Shellfish Immunology 93 (2019) 116–123 Contents lists available at ScienceDirect Fish and Shellfish Immunology journal homepage: www.elsevier.com/locate/fsi Full length article Hesperetin protects crayfish Procambarus clarkii against white spot syndrome virus infection T Xiyi Qian, Fei Zhu* Zhejiang Provincial Engineering Laboratory for Animal Health Inspection and Internet Technology, College of Animal Science and Technology, Zhejiang Agriculture and Forestry University, Hangzhou, 311300, China ARTICLE INFO ABSTRACT Keywords: Hesperetin is a natural flavanone compound, which mainly exists in lemons and oranges, and has potential Hesperetin antiviral and anticancer activities. In this study, hesperetin was used in a crayfish pathogen challenge to discover WSSV its effects on the innate immune system of invertebrates. The crayfish Procambarus clarkii was used as an ex- Innate immunity perimental model and challenged with white spot syndrome virus (WSSV). Pathogen challenge experiments Procambarus clarkii showed that hesperetin treatment significantly reduced the mortality caused by WSSV infection, while the VP28 copies of WSSV were also reduced. Quantitative reverse transcriptase polymerase chain reaction revealed that hesperetin increased the expression of several innate immune-related genes, including NF-kappaB and C-type lectin. Further analysis showed that hesperetin treatment plays a positive effects on three immune parameters like total hemocyte count, phenoloxidase and superoxide dismutase activity. Nevertheless, whether or not in- fected with WSSV, hesperetin treatment would significantly increase the hemocyte apoptosis rates in crayfish. These results indicated that hesperetin could regulate the innate immunity of crayfish, and delaying and re- ducing the mortality after WSSV challenge. Therefore, the present study provided novel insights into the po- tential therapeutic or preventive functions associated with hesperetin to regulate crayfish immunity and protect crayfish against WSSV infection, provide certain theoretical basis for production practice. -

Human Astrovirus 1–8 Seroprevalence Evaluation in a United States Adult Population
UC Santa Cruz UC Santa Cruz Previously Published Works Title Human Astrovirus 1-8 Seroprevalence Evaluation in a United States Adult Population. Permalink https://escholarship.org/uc/item/9nz336gs Journal Viruses, 13(6) ISSN 1999-4915 Authors Meyer, Lena Delgado-Cunningham, Kevin Lorig-Roach, Nicholas et al. Publication Date 2021-05-25 DOI 10.3390/v13060979 Peer reviewed eScholarship.org Powered by the California Digital Library University of California viruses Article Human Astrovirus 1–8 Seroprevalence Evaluation in a United States Adult Population Lena Meyer , Kevin Delgado-Cunningham, Nicholas Lorig-Roach, Jordan Ford and Rebecca M. DuBois * Department of Biomolecular Engineering, University of California Santa Cruz, Santa Cruz, CA 95064, USA; [email protected] (L.M.); [email protected] (K.D.-C.); [email protected] (N.L.-R.); [email protected] (J.F.) * Correspondence: [email protected] Abstract: Human astroviruses are an important cause of viral gastroenteritis globally, yet few studies have investigated the serostatus of adults to establish rates of previous infection. Here, we applied biolayer interferometry immunosorbent assay (BLI-ISA), a recently developed serosurveillance technique, to measure the presence of blood plasma IgG antibodies directed towards the human astrovirus capsid spikes from serotypes 1–8 in a cross-sectional sample of a United States adult population. The seroprevalence rates of IgG antibodies were 73% for human astrovirus serotype 1, 62% for serotype 3, 52% for serotype 4, 29% for serotype 5, 27% for serotype 8, 22% for serotype 2, 8% for serotype 6, and 8% for serotype 7. Notably, seroprevalence rates for capsid spike antigens correlate with neutralizing antibody rates determined previously. -

Virus-Associated RNA I–Deleted Adenovirus, a Potential Oncolytic Agent Targeting EBV-Associated Tumors
Research Article Virus-Associated RNA I–Deleted Adenovirus, a Potential Oncolytic Agent Targeting EBV-Associated Tumors Yaohe Wang,1 Shao-An Xue,2 Gunnel Hallden,1 Jennelle Francis,1 Ming Yuan,1 Beverly E. Griffin,2,3 and Nick R. Lemoine1 1Cancer Research UK Molecular Oncology Unit, Institute of Cancer, Barts and the London School of Medicine and Dentistry, Queen Mary University of London; 2Division of Medicine, Hammersmith Hospital, Imperial College London; and 3Imperial College London at St. Mary’s, London, United Kingdom Abstract More recent reports have linked EBV with conventional epithelial Given the growing number of tumor types recognizably cancers of other sites, including breast (3–5), lung (6–9), prostate associated with EBV infection, it is critically important that (7), liver (10), colon (7), and also with lymphoepithelioma-like therapeutic strategies are developed to treat such tumors. carcinoma of the esophagus (11). Given the ever-growing Replication-selective oncolytic adenoviruses represent number of tumor types associated with EBV infection, thera- a promising new platform for anticancer therapy. Virus- peutic strategies to treat EBV-associated tumors may be associated I (VAI) RNAs of adenoviruses are required for considered a high priority in oncology. efficient translation of viral mRNAs. When the VAI gene is Replication-selective, oncolytic viruses provide a new platform deleted, adenovirus replication is impeded in most cells to treat cancer. Promising clinical trial data with mutant (including HEK 293 cells). EBV-encoded small RNA1 is adenoviruses have shown both their antitumor potency and uniformly expressed in most EBV-associated human tumors safety (12, 13). Two main approaches are currently being used to and can functionally substitute for the VAI RNAs of engineer adenoviruses with tumor-selective replication. -

Survival of Human Norovirus Surrogates in Juices and Their Inactivation Using Novel Methods
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2011 Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods Katie Marie Horm [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Recommended Citation Horm, Katie Marie, "Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods. " Master's Thesis, University of Tennessee, 2011. https://trace.tennessee.edu/utk_gradthes/882 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Katie Marie Horm entitled "Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Food Science and Technology. Doris H. D'Souza, Major Professor We have read this thesis and recommend its acceptance: Federico M. Harte, Gina M. Pighetti Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Katie Marie Horm May 2011 Acknowledgments I would like to think my major professor/advisor Dr. -

Follow-Up of COVID-19 Recovered Patients with Mild Disease
www.nature.com/scientificreports OPEN Follow‑up of COVID‑19 recovered patients with mild disease Alina Kashif1, Manahil Chaudhry2, Tehreem Fayyaz1, Mohammad Abdullah1*, Ayesha Malik2, Javairia Manal Akmal Anwer1, Syed Hashim Ali Inam1, Tehreem Fatima2, Noreena Iqbal3 & Khadija Shoaib1 COVID‑19 may manifest as mild, moderate or severe disease with each grade of severity having its own features and post‑viral implications. With the rising burden of the pandemic, it is vital to identify not only active disease but any post‑recovery complications as well. This study was conducted with the aim of identifying the presence of post‑viral symptomatology in patients recovered from mild COVID‑19 disease. Presence or absence of 11 post‑viral symptoms was recorded and we found that 8 of the 11 studied symptoms were notably more prevalent amongst the female sample population. Our results validate the presence of prolonged symptoms months after recovery from mild COVID‑19 disease, particularly in association with the female gender. Hence, proving the post‑COVID syndrome is a recognizable diagnosis in the bigger context of the post‑viral fatigue syndrome. Te SARS-CoV-2 virus has led to a global health crisis ever since the frst case of COVID-19 was reported in November 2012 in Wuhan, China1. COVID-19 primarily targets the respiratory system with variable initial symptoms including fever, sore throat, fu-like illness, and diarrhea 2. Tere is a chance that some symptoms may linger even afer the convalescence phase has subsided. Te presence of symptoms afer recovery from a viral disease is broadly recognized as a post-viral syndrome3.