Complex Serological Procedures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Allergy Outline
Allergy Evaluation-What it all Means & Role of Allergist Sai R. Nimmagadda, M.D.. Associated Allergists and Asthma Specialists Ltd. Clinical Assistant Professor Of Pediatrics Northwestern University Chicago, Illinois Objectives of Presentation • Discuss the different options for allergy evaluation. – Skin tests – Immunocap Testing • Understand the results of Allergy testing in various allergic diseases. • Briefly Understand what an Allergist Does Common Allergic Diseases Seen in the Primary Care Office • Atopic Dermatitis/Eczema • Food Allergy • Allergic Rhinitis • Allergic Asthma • Allergic GI Diseases Factors that Influence Allergies Development and Expression Host Factors Environmental Factors . Genetic Indoor allergens - Atopy Outdoor allergens - Airway hyper Occupational sensitizers responsiveness Tobacco smoke . Gender Air Pollution . Obesity Respiratory Infections Diet © Global Initiative for Asthma Why Perform Allergy Testing? – Confirm Allergens and answer specific questions. • Am I allergic to my dog? • Do I have a milk allergy? • Have I outgrown my allergy? • Do I need medications? • Am I penicillin allergic? • Do I have a bee sting allergy Tests Performed in the Diagnostic Allergy Laboratory • Allergen-specific IgE (over 200 allergen extracts) – Pollen (weeds, grasses, trees), – Epidermal, dust mites, molds, – Foods, – Venoms, – Drugs, – Occupational allergens (e.g., natural rubber latex) • Total Serum IgE (anti-IgE; ABPA) • Multi-allergen screen for IgE antibody Diagnostic Allergy Testing Serological Confirmation of Sensitization History of RAST Testing • RAST (radioallergosorbent test) invented and marketed in 1974 • The suspected allergen is bound to an insoluble material and the patient's serum is added • If the serum contains antibodies to the allergen, those antibodies will bind to the allergen • Radiolabeled anti-human IgE antibody is added where it binds to those IgE antibodies already bound to the insoluble material • The unbound anti-human IgE antibodies are washed away. -
Syllabus for M
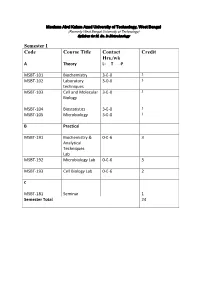
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

Laboratory Techniques Used for Immunological Laboratory Methods
Laboratory techniques used for Immunological laboratory methods Dr. Tatiana Jones, MD, PhD NCC How to Make Serial Dilutions? Interpretation can be made differently depending on the nature of test. For example, if we need to figure out in what sample the concentration of the antibody or antigen is higher, we will go by TITER, which is the lowest serial dilution (let’s say that it is 1:32 in the picture on the left) that gives us positive result. This mean that even diluted 32 times sample is still capable of reacting. The other scenario when we are interpreting quantitative assays, such as ELISA. In this case we need to match results of our samples to known concentrations of STANDARD and MULTIPLY be our dilution factor. What is Antibody Titer? An antibody titer is a measurement of how much antibody an organism has produced that recognizes a particular antigen. Titer is expressed as the inverse of the greatest dilution that still gives a positive result. ELISA is a common means of determining antibody titers. How to Determine Antibody Titer? Where we can use Indirect Coombs test detects the presence of anti-Rh antibodies in blood serum. A patient might be reported to have an "indirect Antibody Titer? Coombs titer" of 16. This means that the patient's serum gives a positive indirect Coombs test at any dilution down to 1/16 (1 part serum to 15 parts diluent). At greater dilutions the indirect Coombs test is negative. If a few weeks later the same patient had an indirect Coombs titer of 32 (1/32 dilution which is 1 part serum to 31 parts diluent), this would mean that more anti-Rh antibody was made, since it took a greater dilution to eradicate the positive test. -

Radial Immunodiffusion Assay Protocol
Radial Immunodiffusion Aim: To study the immunodiffusion technique by Single Radial Immunodiffusion. Introduction: Single Radial Immunodiffusion, also known as Mancini technique, is a quantitative immunodiffusion technique used to detect the concentration of antigen by measuring the diameter of the precipitin ring formed by the interaction of the antigen and the antibody at optimal concentration. In this method the antibody is incorporated into the agarose gel whereas the antigen diffuses into it in a radial pattern. Thus, the antibody is uniformly distributed throughout the gel. Principle: Single Radial Immunodiffusion is used extensively for the quantitative estimation of antigen. Here the antigen-antibody reaction is made more sensitive by the addition of antiserum into the agarose gel and loading the antigen sample in the well. As the antigen diffuses into the agarose radially in all directions, it’s concentration continuously falls until the equivalence point is reached at which the antigen concentration is in equal proportion to that of the antibody present in the agarose gel. At this point ring of precipitation (‘precipitin ring’) is formed around the well. The diameter of the precipitin ring is proportional to the concentration of antigen. With increasing concentration of antigen, precipitin rings with larger diameter are formed. The size of the precipitin rings depends on: Antigen concentration in the sample well Antibody concentration in the agarose gel Size of the sample well Volume of the sample Thus, by having various concentrations of a standard antigen, standard curve can be obtained from which one can determine the amount of an antigen in an unknown sample. Thus, this is a quantitative test. -

An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1981 An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate Allen H. Smith Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biochemistry Commons Recommended Citation Smith, Allen H., "An Enzyme-Linked Immunosorbent Assay (ELISA) for Pantothenate" (1981). All Graduate Theses and Dissertations. 5295. https://digitalcommons.usu.edu/etd/5295 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. AN ENZYME- LINKED ThltviUNOSORBE!'IT ASSAY (ELISA) FOR PANTOTHENATE by Allen H. Smith A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biochemistry UTAH STATE UNIVERSITY Logan, Utah 1981 ii ACKNOWLEDGEMENTS To Dr. R. G. Hansen, to Dr. B. W. Wyse, to Carl Wittwer, to Jack Brown, to Jan Pearson, to Nedra Christensen, to all those who have made this experience one of tremendous growth, I express my thanks. I express appreciation to the United States Department of Agriculture, under Grant #5901-0410-9-0288-0 with Utah State University, for financial support. Finally, I express thanks to my parents, who have come to realize that graduate school is also a part of life. Allen H. Smith "iii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ii LIST OF TABLES . vi LIST OF FIGURES. vii · ABSTRACT .. ix INTRODUCTION 1 REVIEW OF LITERATURE 4 Pantothenate Assays . -

RAST Type Tests CPT: 86003
Medicare Local Coverage Determination Policy Allergy Testing RAST Type Tests CPT: 86003 Medically Supportive CMS Policy for Kentucky and Ohio ICD Codes are listed Local policies are determined by the performing test location. This is determined by the state on subsequent page(s) in which your performing laboratory resides and where your testing is commonly performed. of this document. Coverage Indications, Limitations, and/or Medical Necessity Radioallergosorbent test (RAST), fluoroallergosorbent test (FAST), and multiple antigen simultaneous tests are in vitro techniques for Add full policy information determining whether a patient's serum contains IgE antibodies against specific allergens of clinical importance. As with any allergy testing, the need for such tests is based on the findings during a complete history and physical examination of the patient. The multiple antigen simultaneous testing technique is similar to the RAST/FAST techniques in that it depends upon the existence of Template structure: allergic antibodies in the blood of the patient being tested. With the multiple antigen simultaneous test system, several antigens may be used to test for specific IgE simultaneously. First level is for headers such as limitations, ELISA (enzyme-linked immunosorbent assay) is another in vitro method of allergy testing for specific IgE antibodies against allergens. indications and usage guidelines This method is also a variation of RAST. Second level is for main body copy Limitations • Third level is for bullet (if needed) The following tests are considered to be not medically necessary and will be denied. • ELISA/Act qualitative antibody testing. This testing is used to determine in vitro reaction to various foods and relies on lymphocyte blastogenesis in response to certain food antigens. -

Red Blood Cell Preparation and Hemagglutination Assay
Red Blood Cell Preparation and Hemagglutination Assay Purpose: This protocol describes the preparation of RBCs for storage and use in Hemagluttination assays, which are used to determine the La Sota B1 lentogenic Newcastle Disease virion hemaglutinin-neuraminidase titer relative to virion stock positive controls. The protocol was adapted from McGinnes et al. (2006) and “Detection of Hemagglutinating Viruses” (n.d.). Materials: ● Whole Blood Cells ● 50mL conical tube ● Multichannel micropipette, micropipette, and tips ● PBS with 2mM Penicillin/Streptomycin ● SV3 ● Alsever’s Solution ● 96 round bottom well plate ● Microscope slide ● Virion stock ● Centrifuge Procedure: 1. RBC preparation a. Obtain 25mL whole blood and add 25mL cold Alsever’s Solution for anti-coagulation in a 50mL conical tube and keep on ice b. Centrifuge whole blood at 500 RCF for 10min c. Aspirate blood plasma, buffy layer, and top erythrocytes d. Wash 3 times by resuspending in PBS with 2mM Penicillin/Streptomycin to double the pellet volume, centrifuging at 500 RCF, and aspirating the supernatant e. Resuspend the pellet in PBS with 2mM Penicillin/Streptomycin or SV3 for a final concentration of 10% pellet volume per total volume, and store at 2-7˚C for 1 week or 42 days 2. HA Titer a. Thaw your virion stock and samples on ice b. Use a multichannel micropipette to add 50µL of cold PBS to each row on a 96 round bottom well plate for each sample in duplicates c. Add 50µL of each sample to column 1 of their respective duplicate rows i. Don’t add anything to the negative control rows and add virion stocks to the positive control rows d. -

Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A
JOURNAL OF CLINICAL MICROBIOLOGY, June 1976, p. 637-639 Vol. 3, No. 6 Copyright © 1976 American Society for Microbiology Printed in U.S.A. Detection of Virus-Specific Immunoglobulins Using a Doubly Labeled Fluorescein- 125I Antibody A. J. PARKINSON* AND J. KALMAKOFF Department ofMicrobiology, University of Otago, Dunedin, New Zealand Received for publication 17 February 1976 Commercially prepared fluorescein-labeled antihuman antibodies were la- beled with 125I and used to compare specific herpes simplex virus antibody titers as determined by indirect fluorescent antibody and radioimmunoassay tech- niques. Total virus-specific immunoglobulin and virus-specific immunoglobulin G titers did not vary by more than one twofold dilution when compared by the two methods. Efforts are being made to develop a reliable calf serum, penicillin (100 U/ml) streptomycin radioimmunoassay (RIA) for the detection of (100 ,ug/ml), and 0.1% bicarbonate, were in- virus-specific immunoglobulins, acceptable for fected with the isolated virus. Uninoculated use in diagnostic serology (1, 2, 5-8). The estab- monolayers were maintained as controls. When lishment of a satisfactory RIA depends on the infected monolayers showed 75% cytopathic use of antibody with both a high avidity and effect, both inoculated and uninoculated cells selectivity for the material to be assayed (4). were dispersed, using 0.015% ethylenediamine- Consequently, a major obstacle to using RIA tetraacetic acid. Both cellular suspensions were routinely is the necessity of preparing specific standardized to contain 2.5 x 105 cells/ml in high-titer antibody against human immuno- phosphate-buffered saline. Using 0.025-ml vol- globulins (IgG, IgM, and IgA). -

Importance of Ag-Ab Reactions
Ag-Ab reactions Tests for Ag-Ab reactions EISA SALEHI PhD. Immunology Dept. TUMS Importance of Ag-Ab Reactions • Understand the mechanisms of defense • Abs as tools in: – Treatment – Diagnosis • As biomarkers • As tools to measure analytes Nature of Ag/Ab Reactions http://www.med.sc.edu:85/chime2/lyso-abfr.htm • Lock and Key Concept • Non-covalent Bonds – Hydrogen bonds – Electrostatic bonds – Van der Waal forces – Hydrophobic bonds • Multiple Bonds • Reversible Source: Li, Y., Li, H., Smith-Gill, S. J., Mariuzza, R. A., Biochemistry 39, 6296, 2000 Affinity • Strength of the reaction between a single antigenic determinant and a single Ab combining site High Affinity Low Affinity Ab Ab Ag Ag Affinity = ( attractive and repulsive forces Calculation of Affinity Ag + Ab ↔ Ag-Ab Applying the Law of Mass Action: [[gAg-Ab] Keq = [Ag] x [Ab] Avidity • The overall strength of binding between an Ag with many determinants and multivalent Abs 4 6 10 Keq = 10 10 10 Affinity Avidity Avidity SifiitSpecificity • The ability of an individual antibody combining site to react with only one antigenic determinant. • The ability of a population of antibody molecules to react with only one antigen. Cross Reactivity • The ability of an individual Ab combining site to react with more than one antigenic determinant. • The ability of a population of Ab molecules to react with more than one Ag Cross reactions Anti-A Anti-A Anti-A Ab Ab Ab Ag A Ag B Ag C Shared epitope Similar epitope Factors Affecting Measurement of A/AbRAg/Ab Reac tions • Affinity • Avidity Ab excess Ag excess • AAbiAg:Ab ratio •Phyygsical form of Ag Equivalence – Lattice formation Do you need to know what happens in Lab. -

Radioallergosorbent Test (RAST)—Reliable Tool Or Poor Substitute?
Radioallergosorbent test (RAST) VII Ö1 1 reliable tool or poor substitute? Edward W. Hein, M.D. An in vitro method, the radioallergosorbent test (RAST) has been developed for the detection of allergen-specific antibodies of the IgE class. Review of the literature shows that in comparison to skin testing, the RAST has a high degree of correlation (60% to 90% depending on the antigen); however, this method is not as sensitive as other tests (50% false-negative). The RAST is affected by blocking antibodies (IgG), resulting in false-negative values and high levels of IgE that bind on the allergen discs, giving false- positive findings. Because of these problems, RAST is somewhat limited for use in the clinical setting. Index terms: Allergy and immunology • Radioallergosor- bent test (RAST) Cleve Clin Q 50:361-366, Fall 1983 Skin testing has been the traditional method for diagnosis of IgE-mediated allergic disorders. This bioassay is highly sensitive, cost-effective, and safe when used by experienced personnel. In 1967, Wide et al,1 in Sweden, reported a new technique capable of detecting the minute quantities of allergen-specific IgE antibodies that circulate in the serum of allergic patients. This laboratory procedure, called RAST (radioallergosorbent test), utilized a solid- phase radioimmunoassay method. During the last decade this in vitro test has been refined and is now a commercially 1 Departments of Pediatric and Adolescent Medi- available laboratory test for clinical laboratories and, in kit cine, and Allergy and Immunology, The Cleveland form, for physicians' offices. Proponents of this new Clinic Foundation. (E.W.H., Head, Section Pediatric method claim that its results are more objective, safer for Allergy and Clinical Immunology.) Submitted for pub- lication May 1983; accepted June 1983. -

Hypersensitivity Reactions (Types I, II, III, IV)
Hypersensitivity Reactions (Types I, II, III, IV) April 15, 2009 Inflammatory response - local, eliminates antigen without extensively damaging the host’s tissue. Hypersensitivity - immune & inflammatory responses that are harmful to the host (von Pirquet, 1906) - Type I Produce effector molecules Capable of ingesting foreign Particles Association with parasite infection Modified from Abbas, Lichtman & Pillai, Table 19-1 Type I hypersensitivity response IgE VH V L Cε1 CL Binds to mast cell Normal serum level = 0.0003 mg/ml Binds Fc region of IgE Link Intracellular signal trans. Initiation of degranulation Larche et al. Nat. Rev. Immunol 6:761-771, 2006 Abbas, Lichtman & Pillai,19-8 Factors in the development of allergic diseases • Geographical distribution • Environmental factors - climate, air pollution, socioeconomic status • Genetic risk factors • “Hygiene hypothesis” – Older siblings, day care – Exposure to certain foods, farm animals – Exposure to antibiotics during infancy • Cytokine milieu Adapted from Bach, JF. N Engl J Med 347:911, 2002. Upham & Holt. Curr Opin Allergy Clin Immunol 5:167, 2005 Also: Papadopoulos and Kalobatsou. Curr Op Allergy Clin Immunol 7:91-95, 2007 IgE-mediated diseases in humans • Systemic (anaphylactic shock) •Asthma – Classification by immunopathological phenotype can be used to determine management strategies • Hay fever (allergic rhinitis) • Allergic conjunctivitis • Skin reactions • Food allergies Diseases in Humans (I) • Systemic anaphylaxis - potentially fatal - due to food ingestion (eggs, shellfish,